Anti-selective [3+2] (Hetero)annulation of non-conjugated alkenes via directed nucleopalladation
- PMID: 33353940
- PMCID: PMC7755910
- DOI: 10.1038/s41467-020-20182-4
Anti-selective [3+2] (Hetero)annulation of non-conjugated alkenes via directed nucleopalladation
Abstract
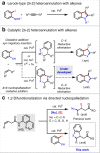
2,3-Dihydrobenzofurans and indolines are common substructures in medicines and natural products. Herein, we describe a method that enables direct access to these core structures from non-conjugated alkenyl amides and ortho-iodoanilines/phenols. Under palladium(II) catalysis this [3 + 2] heteroannulation proceeds in an anti-selective fashion and tolerates a wide variety of functional groups. N-Acetyl, -tosyl, and -alkyl substituted ortho-iodoanilines, as well as free -NH2 variants, are all effective. Preliminary results with carbon-based coupling partners also demonstrate the viability of forming indane core structures using this approach. Experimental and computational studies on reactions with phenols support a mechanism involving turnover-limiting, endergonic directed oxypalladation, followed by intramolecular oxidative addition and reductive elimination.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Liu D, Zhao G, Xiang L. Diverse strategies for the synthesis of the indoline scaffold. Eur. J. Org. Chem. 2010;2010:3975–3984. doi: 10.1002/ejoc.201000323. - DOI
-
- Silva TS, et al. Recent advances in Indoline synthesis. Tetrahedron. 2019;75:2063–2097. doi: 10.1016/j.tet.2019.02.006. - DOI
-
- Bertolini F, Pineschi M. Recent progress in the synthesis of 2,3-dihydrobenzofurans. Org. Prep. Proc. Int. 2009;41:385–418. doi: 10.1080/00304940903240836. - DOI
-
- Larock RC, Yum EK. Synthesis of indoles via palladium-catalyzed heteroannulation of internal alkynes. J. Am. Chem. Soc. 1991;113:6689–6690. doi: 10.1021/ja00017a059. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

