Expanding the antibacterial selectivity of polyether ionophore antibiotics through diversity-focused semisynthesis
- PMID: 33353970
- PMCID: PMC7610524
- DOI: 10.1038/s41557-020-00601-1
Expanding the antibacterial selectivity of polyether ionophore antibiotics through diversity-focused semisynthesis
Abstract
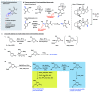
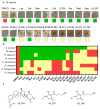
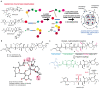
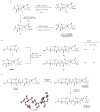
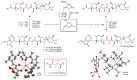
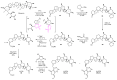
Polyether ionophores are complex natural products capable of transporting cations across biological membranes. Many polyether ionophores possess potent antimicrobial activity and a few selected compounds have the ability to target aggressive cancer cells. Nevertheless, ionophore function is believed to be associated with idiosyncratic cellular toxicity and, consequently, human clinical development has not been pursued. Here, we demonstrate that structurally novel polyether ionophores can be efficiently constructed by recycling components of highly abundant polyethers to afford analogues with enhanced antibacterial selectivity compared to a panel of natural polyether ionophores. We used classic degradation reactions of the natural polyethers lasalocid and monensin and combined the resulting fragments with building blocks provided by total synthesis, including halogen-functionalized tetronic acids as cation-binding groups. Our results suggest that structural optimization of polyether ionophores is possible and that this area represents a potential opportunity for future methodological innovation.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Schreiber SL. Target-oriented and diversity-oriented organic synthesis in drug discovery. Science. 2000;287:1964–1969. - PubMed
-
- Wetzel S, Bon RS, Kumar K, Waldmann H. Biology-oriented synthesis. Angew Chem Int Ed. 2011;50:10800–10826. - PubMed
-
- Wilson RM, Danishefsky SJ. Small molecule natural products in the discovery of therapeutic agents: the synthesis connection. J Org Chem. 2006;71:8329–8351. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

