Critical Analysis of a Randomized Controlled Trial
- PMID: 33354045
- PMCID: PMC7724939
- DOI: 10.5005/jp-journals-10071-23638
Critical Analysis of a Randomized Controlled Trial
Abstract
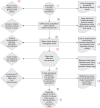
In the era of evidence-based medicine, healthcare professionals are bombarded with plenty of trials and articles of which randomized control trial is considered as the epitome of all in terms of level of evidence. It is very crucial to learn the skill of balancing knowledge of randomized control trial and to avoid misinterpretation of trial result in clinical practice. There are various methods and steps to critically appraise the randomized control trial, but those are overly complex to interpret. There should be more simplified and pragmatic approach for analysis of randomized controlled trial. In this article, we like to summarize few of the practical points under 5 headings: "5 'Rs' of critical analysis of randomized control trial" which encompass Right Question, Right Population, Right Study Design, Right Data, and Right Interpretation. This article gives us insight that analysis of randomized control trial should not only based on statistical findings or results but also on systematically reviewing its core question, relevant population selection, robustness of study design, and right interpretation of outcome. How to cite this article: Nimavat BD, Zirpe KG, Gurav SK. Critical Analysis of a Randomized Controlled Trial. Indian J Crit Care Med 2020;24(Suppl 4):S215-S222.
Keywords: Critical analysis; Evidence based medicine; Randomized control trial.
Copyright © 2020; Jaypee Brothers Medical Publishers (P) Ltd.
Conflict of interest statement
Source of support: Nil Conflict of interest: None
Figures
References
-
- Veldhoen RA, Howes D, Maslove DM. Is mortality a useful primary end point for critical care trials? Chest, Elsevier Inc. 2020;158(1):206–211. - PubMed
-
- “Endpoints used for Relative Effectiveness Assessment of pharmaceuticals: Clinical Endpoints”-February 2013 GUIDELINE Endpoints used for Relative Effectiveness Assessment: Clinical Endpoints cki.
-
- fda. Clinical Trial Endpoints.
Publication types
LinkOut - more resources
Full Text Sources