This is a preprint.
Real-world data suggest antibody positivity to SARS-CoV-2 is associated with a decreased risk of future infection
- PMID: 33354682
- PMCID: PMC7755144
- DOI: 10.1101/2020.12.18.20248336
Real-world data suggest antibody positivity to SARS-CoV-2 is associated with a decreased risk of future infection
Update in
-
Association of SARS-CoV-2 Seropositive Antibody Test With Risk of Future Infection.JAMA Intern Med. 2021 May 1;181(5):672-679. doi: 10.1001/jamainternmed.2021.0366. JAMA Intern Med. 2021. PMID: 33625463 Free PMC article.
Abstract
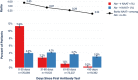
Importance There is limited evidence regarding whether the presence of serum antibodies to SARS-CoV-2 is associated with a decreased risk of future infection. Understanding susceptibility to infection and the role of immune memory is important for identifying at-risk populations and could have implications for vaccine deployment. Objective The purpose of this study was to evaluate subsequent evidence of SARS-CoV-2 infection based on diagnostic nucleic acid amplification test (NAAT) among individuals who are antibody-positive compared with those who are antibody-negative, using real-world data. Design This was an observational descriptive cohort study. Participants The study utilized a national sample to create cohorts from a de-identified dataset composed of commercial laboratory test results, open and closed medical and pharmacy claims, electronic health records, hospital billing (chargemaster) data, and payer enrollment files from the United States. Patients were indexed as antibody-positive or antibody-negative according to their first SARS-CoV-2 antibody test recorded in the database. Patients with more than 1 antibody test on the index date where results were discordant were excluded. Main Outcomes/Measures Primary endpoints were index antibody test results and post-index diagnostic NAAT results, with infection defined as a positive diagnostic test post-index, as measured in 30-day intervals (0-30, 31-60, 61-90, >90 days). Additional measures included demographic, geographic, and clinical characteristics at the time of the index antibody test, such as recorded signs and symptoms or prior evidence of COVID-19 (diagnoses or NAAT+) and recorded comorbidities. Results We included 3,257,478 unique patients with an index antibody test. Of these, 2,876,773 (88.3%) had a negative index antibody result, 378,606 (11.6%) had a positive index antibody result, and 2,099 (0.1%) had an inconclusive index antibody result. Patients with a negative antibody test were somewhat older at index than those with a positive result (mean of 48 versus 44 years). A fraction (18.4%) of individuals who were initially seropositive converted to seronegative over the follow up period. During the follow-up periods, the ratio (CI) of positive NAAT results among individuals who had a positive antibody test at index versus those with a negative antibody test at index was 2.85 (2.73 - 2.97) at 0-30 days, 0.67 (0.6 - 0.74) at 31-60 days, 0.29 (0.24 - 0.35) at 61-90 days), and 0.10 (0.05 - 0.19) at >90 days. Conclusions Patients who display positive antibody tests are initially more likely to have a positive NAAT, consistent with prolonged RNA shedding, but over time become markedly less likely to have a positive NAAT. This result suggests seropositivity using commercially available assays is associated with protection from infection. The duration of protection is unknown and may wane over time; this parameter will need to be addressed in a study with extended duration of follow up.
Figures


References
-
- Stadlbauer D, Tan J, Jiang K, et al. Repeated cross-sectional sero-monitoring of SARS-CoV-2 in New York City. Nature. Published online 2020:1–7. - PubMed
-
- Ward H, Atchison CJ, Whitaker M, et al. Antibody prevalence for SARS-CoV-2 in England following first peak of the pandemic: REACT2 study in 100,000 adults. medRxiv. Published online January 1, 2020:2020.08.12.20173690. doi: 10.1101/2020.08.12.20173690 - DOI
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
