Clinical Outcomes of 2-Drug Regimens vs 3-Drug Regimens in Antiretroviral Treatment-Experienced People Living With Human Immunodeficiency Virus
- PMID: 33354721
- PMCID: PMC9431658
- DOI: 10.1093/cid/ciaa1878
Clinical Outcomes of 2-Drug Regimens vs 3-Drug Regimens in Antiretroviral Treatment-Experienced People Living With Human Immunodeficiency Virus
Abstract
Background: Limited data exist that compare clinical outcomes of 2-drug regimens (2DRs) and 3-drug regimens (3DRs) in people living with human immunodeficiency virus.
Methods: Antiretroviral treatment-experienced individuals in the International Cohort Consortium of Infectious Diseases (RESPOND) who switched to a new 2DR or 3DR from 1 January 2012-1 October 2018 were included. The incidence of clinical events (AIDS, non-AIDS cancer, cardiovascular disease, end-stage liver and renal disease, death) was compared between regimens using Poisson regression.
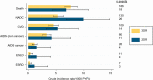
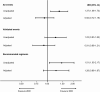
Results: Of 9791 individuals included, 1088 (11.1%) started 2DRs and 8703 (88.9%) started 3DRs. The most common 2DRs were dolutegravir plus lamivudine (22.8%) and raltegravir plus boosted darunavir (19.8%); the most common 3DR was dolutegravir plus 2 nucleoside reverse transcriptase inhibitors (46.9%). Individuals on 2DRs were older (median, 52.6 years [interquartile range, 46.7-59.0] vs 47.7 [39.7-54.3]), and a higher proportion had ≥1 comorbidity (81.6% vs 73.9%). There were 619 events during 27 159 person-years of follow-up (PYFU): 540 (incidence rate [IR] 22.5/1000 PYFU; 95% confidence interval [CI]: 20.7-24.5) on 3DRs and 79 (30.9/1000 PYFU; 95% CI: 24.8-38.5) on 2DRs. The most common events were death (7.5/1000 PYFU; 95% CI: 6.5-8.6) and non-AIDS cancer (5.8/1000 PYFU; 95% CI: 4.9-6.8). After adjustment for baseline demographic and clinical characteristics, there was a similar incidence of events on both regimen types (2DRs vs 3DRs IR ratio, 0.92; 95% CI: .72-1.19; P = .53).
Conclusions: This is the first large, international cohort to assess clinical outcomes on 2DRs. After accounting for baseline characteristics, there was a similar incidence of events on 2DRs and 3DRs. 2DRs appear to be a viable treatment option with regard to clinical outcomes. Further research on resistance barriers and long-term durability of 2DRs is needed.
Keywords: 2-drug regimens; HIV; antiretroviral treatment; clinical outcomes; dual therapy.
© The Author(s) 2020. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail: journals.permissions@oup.com.
Figures

References
-
- EACS . EACS guidelines version 10.1. EACS (European AIDS Clinical Society), 2020. Available at: https://www.eacsociety.org/files/guidelines-10.1_finaljan2021_1.pdf.
-
- Palella FJ Jr, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med 1998; 338:853–60. - PubMed
-
- Fernandez-Montero JV, Eugenia E, Barreiro P, Labarga P, Soriano V. Antiretroviral drug-related toxicities— clinical spectrum, prevention, and management. Expert Opin Drug Saf 2013; 12:697–707. - PubMed

