Impact of Sequencing Depth and Library Preparation on Toxicological Interpretation of RNA-Seq Data in a "Three-Sample" Scenario
- PMID: 33354967
- PMCID: PMC7887111
- DOI: 10.1021/acs.chemrestox.0c00368
Impact of Sequencing Depth and Library Preparation on Toxicological Interpretation of RNA-Seq Data in a "Three-Sample" Scenario
Abstract
While RNA-sequencing (RNA-seq) has emerged as a standard approach in toxicogenomics, its full potential in gaining underlying toxicological mechanisms is still not clear when only three biological replicates are used. This "three-sample" study design is common in toxicological research, particularly in animal studies during preclinical drug development. Sequencing depth (the total number of reads in an experiment) and library preparation are critical to the resolution and integrity of RNA-seq data and biological interpretation. We used aflatoxin b1 (AFB1), a model toxicant, to investigate the effect of sequencing depth and library preparation in RNA-seq on toxicological interpretation in the "three-sample" scenario. We also compared different gene profiling platforms (RNA-seq, TempO-seq, microarray, and qPCR) using identical liver samples. Well-established mechanisms of AFB1 toxicity served as ground truth for our comparative analyses. We found that a minimum of 20 million reads was sufficient to elicit key toxicity functions and pathways underlying AFB1-induced liver toxicity using three replicates and that identification of differentially expressed genes was positively associated with sequencing depth to a certain extent. Further, our results showed that RNA-seq revealed toxicological insights from pathway enrichment with overall higher statistical power and overlap ratio, compared with TempO-seq and microarray. Moreover, library preparation using the same methods was important to reproducing the toxicological interpretation.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
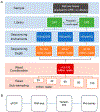
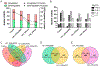





References
-
- Mortazavi A, Williams BA, McCue K, Schaeffer L, and Wold B (2008) Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat. Methods 5, 621–628. - PubMed
-
- Wang C, Gong B, Bushel PR, Thierry-Mieg J, Thierry-Mieg D, Xu J, Fang H, Hong H, Shen J, Su Z, Meehan J, Li X, Yang L, Li H, Labaj PP, Kreil DP, Megherbi D, Gaj S, Caiment F, van Delft J, Kleinjans J, Scherer A, Devanarayan V, Wang J, Yang Y, Qian HR, Lancashire LJ, Bessarabova M, Nikolsky Y, Furlanello C, Chierici M, Albanese D, Jurman G, Riccadonna S, Filosi M, Visintainer R, Zhang KK, Li J, Hsieh JH, Svoboda DL, Fuscoe JC, Deng Y, Shi L, Paules RS, Auerbach SS, and Tong W (2014) The concordance between RNA-seq and microarray data depends on chemical treatment and transcript abundance. Nat. Biotechnol 32, 926–932. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

