Epidemiology and treatment patterns for locally advanced or metastatic urothelial carcinoma: a systematic literature review and gap analysis
- PMID: 33355035
- PMCID: PMC10394179
- DOI: 10.18553/jmcp.2020.20285
Epidemiology and treatment patterns for locally advanced or metastatic urothelial carcinoma: a systematic literature review and gap analysis
Abstract
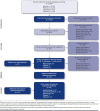
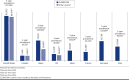
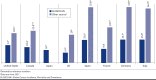
BACKGROUND: Several immuno-oncology (IO) agents targeting programmed death-1 or programmed death-ligand 1 (PD-1/L1) are approved second-line therapy options for patients with locally advanced or metastatic urothelial carcinoma (la/mUC) previously treated with platinum-based chemotherapy or first-line options in patients ineligible for cisplatin whose tumors express PD-L1 or for any platinum-based chemotherapy regardless of PD-L1 expression levels. However, literature on the epidemiology of la/mUC is limited, and real-world treatment patterns are not well established, especially with respect to therapies used following IO. OBJECTIVES: To (a) report the epidemiology of urothelial carcinoma (UC) and la/mUC; (b) identify and summarize the published literature on la/mUC treatment patterns, including IO and post-IO treatment; and (c) identify evidence gaps. METHODS: A systematic literature review was conducted using Cochrane dual-reviewer methodology and the Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols. Literature databases and selected congress abstracts (2017-2018) were searched for retrospective studies published January 2013-August 2018 in English reporting epidemiological and treatment data (all lines of therapy) for adult patients with la/mUC. RESULTS: Among 6,584 database references and 1,832 congress abstracts screened, 45 publications (29 manuscripts, 1 poster, 15 abstracts; reporting 37 unique studies) were retained. All studies related to treatment patterns, and the majority were from the United States (n = 17), Japan (n = 8), and the United Kingdom (n = 5). Epidemiological data were not identified among the searches thus online registries were leveraged. Among the identified publications, 21 (20 unique) reported on cisplatin versus non-cisplatin regimens, 14 (8 unique) on IO, and 9 (7 unique) on vinflunine. Cisplatin use varied both within and among countries (ranging from 18.4% in 1 U.S. study to 87.9% in 1 Japanese study). The use of IO was higher in later lines of therapy, ranging from 1.4% to 7.9% as first-line therapy to 57.8% as second-line and 64.4% as third-line therapy. Among studies reporting IO discontinuation rates, 41.4%-71% of patients were reported to discontinue IO across the studies, and the median time to discontinuation ranged from 2.7 to 5.8 months. Only 25%-35.5% of patients received subsequent therapy following IO discontinuation; post-IO treatments varied widely. CONCLUSIONS: Additional published data on the country-specific epidemiology of UC and la/mUC are needed, including rates of progression from early-stage disease to la/mUC. There was large variation in treatment rates, particularly cisplatin use, within and across countries. The few published real-world IO studies reported high levels of discontinuation with only a small percentage of patients receiving subsequent therapy. As IO therapies continue to be granted regulatory approval in countries outside the United States and novel therapies gain approval in the post-IO setting, the treatment paradigm for patients with la/mUC is shifting, and future studies with more recent data will be required. DISCLOSURES: This study was funded by Astellas/Seagen. Hepp is an employee of and owns stock in Seagen. Shah was a contractor for Astellas Pharma at the time of the study and owns stock in Pfizer. Smoyer is an employee and shareholder of Envision Pharma Group, paid consultants to Seagen. Vadagam was an employee of Envision Pharma Group, paid consultants to Seagen, at the time of the study. Parts of these data have been presented at the International Society for Pharmacoeconomics and Outcomes Research (ISPOR) 2019 Annual Meeting; May 18-22, 2019; New Orleans, LA.
Conflict of interest statement
This study was funded by Astellas/Seagen. Hepp is an employee of and owns stock in Seagen. Shah was a contractor for Astellas Pharma at the time of the study and owns stock in Pfizer. Smoyer is an employee and shareholder of Envision Pharma Group, paid consultants to Seagen. Vadagam was an employee of Envision Pharma Group, paid consultants to Seagen, at the time of the study.
Parts of these data have been presented at the International Society for Pharmacoeconomics and Outcomes Research (ISPOR) 2019 Annual Meeting; May 18-22, 2019; New Orleans, LA.
Figures
References
-
- Ferlay J, Ervik M, Lam F, et al. Cancer today: data visualization tools for exploring the global cancer burden in 2018. 2018. Accessed November 25, 2020. https://gco.iarc.fr/today
-
- Bamias A, Tzannis K, Harshman LC, et al. Impact of contemporary patterns of chemotherapy utilization on survival in patients with advanced cancer of the urinary tract: a Retrospective International Study of Invasive/Advanced Cancer of the Urothelium (RISC). Ann Oncol. 2018;29(2):361-69. - PMC - PubMed
-
- Fleshner NE, Herr HW, Stewart AK, Murphy GP, Mettlin C, Menck HR. The National Cancer Data Base report on bladder carcinoma: the American College of Surgeons Commission on Cancer and the American Cancer Society. Cancer. 1996;78(7):1505-13. - PubMed
-
- Boustead GB, Fowler S, Swamy R, Kocklebergh R, Hounsome L; Section of Oncology-BAUS. Stage, grade and pathological characteristics of bladder cancer in the UK: British Association of Urological Surgeons (BAUS) urological tumour registry. BJU Int. 2014;113(6):924-30. - PubMed
-
- National Cancer Institute Surveillance, Epidemiology, and End Results Program. Cancer stat facts: bladder cancer. 2020. Accessed November 25, 2020. https://seer.cancer.gov/statfacts/html/urinb.html
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous