Termini restraining of small membrane proteins enables structure determination at near-atomic resolution
- PMID: 33355146
- PMCID: PMC11205269
- DOI: 10.1126/sciadv.abe3717
Termini restraining of small membrane proteins enables structure determination at near-atomic resolution
Abstract
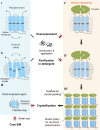
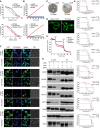
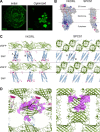
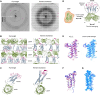
Small membrane proteins are difficult targets for structural characterization. Here, we stabilize their folding by restraining their amino and carboxyl termini with associable protein entities, exemplified by the two halves of a superfolder GFP. The termini-restrained proteins are functional and show improved stability during overexpression and purification. The reassembled GFP provides a versatile scaffold for membrane protein crystallization, enables diffraction to atomic resolution, and facilitates crystal identification, phase determination, and density modification. This strategy gives rise to 14 new structures of five vertebrate proteins from distinct functional families, bringing a substantial expansion to the structural database of small membrane proteins. Moreover, a high-resolution structure of bacterial DsbB reveals that this thiol oxidoreductase is activated through a catalytic triad, similar to cysteine proteases. Overall, termini restraining proves exceptionally effective for stabilization and structure determination of small membrane proteins.
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works. Distributed under a Creative Commons Attribution NonCommercial License 4.0 (CC BY-NC).
Figures

Similar articles
-
Distinct enzymatic strategies for de novo generation of disulfide bonds in membranes.Crit Rev Biochem Mol Biol. 2023 Feb;58(1):36-49. doi: 10.1080/10409238.2023.2201404. Epub 2023 Apr 25. Crit Rev Biochem Mol Biol. 2023. PMID: 37098102 Free PMC article.
-
Stabilization and structure determination of integral membrane proteins by termini restraining.Nat Protoc. 2022 Feb;17(2):540-565. doi: 10.1038/s41596-021-00656-5. Epub 2022 Jan 17. Nat Protoc. 2022. PMID: 35039670 Free PMC article. Review.
-
Cloning, expression, crystallization and preliminary X-ray studies of a superfolder GFP fusion of cyanobacterial Psb32.Acta Crystallogr F Struct Biol Commun. 2015 Apr;71(Pt 4):409-13. doi: 10.1107/S2053230X15003970. Epub 2015 Mar 20. Acta Crystallogr F Struct Biol Commun. 2015. PMID: 25849501 Free PMC article.
-
The mechanism of folding robustness revealed by the crystal structure of extra-superfolder GFP.FEBS Lett. 2017 Jan;591(2):442-447. doi: 10.1002/1873-3468.12534. Epub 2016 Dec 28. FEBS Lett. 2017. PMID: 27990640
-
Advanced solid-state NMR approaches for structure determination of membrane proteins and amyloid fibrils.Acc Chem Res. 2013 Sep 17;46(9):2080-8. doi: 10.1021/ar4000168. Epub 2013 May 10. Acc Chem Res. 2013. PMID: 23659727 Review.
Cited by
-
Lateral Habenula Neurons Signal Cold Aversion and Participate in Cold Aversion.Neurochem Res. 2024 Mar;49(3):771-784. doi: 10.1007/s11064-023-04076-7. Epub 2023 Dec 16. Neurochem Res. 2024. PMID: 38102342
-
Carbon dioxide photoreduction in prebiotic environments.Photochem Photobiol Sci. 2022 May;21(5):863-878. doi: 10.1007/s43630-021-00168-x. Epub 2022 Feb 2. Photochem Photobiol Sci. 2022. PMID: 35107790 Review.
-
Recent advances of photoresponsive nanomaterials for diagnosis and treatment of acute kidney injury.J Nanobiotechnology. 2024 Nov 5;22(1):676. doi: 10.1186/s12951-024-02906-6. J Nanobiotechnology. 2024. PMID: 39501286 Free PMC article. Review.
-
Distinct enzymatic strategies for de novo generation of disulfide bonds in membranes.Crit Rev Biochem Mol Biol. 2023 Feb;58(1):36-49. doi: 10.1080/10409238.2023.2201404. Epub 2023 Apr 25. Crit Rev Biochem Mol Biol. 2023. PMID: 37098102 Free PMC article.
-
The transcriptome of anterior regeneration in earthworm Eudrilus eugeniae.Mol Biol Rep. 2021 Jan;48(1):259-283. doi: 10.1007/s11033-020-06044-8. Epub 2020 Dec 11. Mol Biol Rep. 2021. PMID: 33306150
References
-
- Kang H. J., Lee C., Drew D., Breaking the barriers in membrane protein crystallography. Int. J. Biochem. Cell Biol. 45, 636–644 (2013). - PubMed
-
- Tate C. G., A crystal clear solution for determining G-protein-coupled receptor structures. Trends Biochem. Sci. 37, 343–352 (2012). - PubMed
-
- Magnani F., Serrano-Vega M. J., Shibata Y., Abdul-Hussein S., Lebon G., Miller-Gallacher J., Singhal A., Strege A., Thomas J. A., Tate C. G., A mutagenesis and screening strategy to generate optimally thermostabilized membrane proteins for structural studies. Nat. Protoc. 11, 1554–1571 (2016). - PMC - PubMed
-
- Zhou Y., Morais-Cabral J. H., Kaufman A., MacKinnon R., Chemistry of ion coordination and hydration revealed by a K+ channel–Fab complex at 2.0 Å resolution. Nature 414, 43–48 (2001). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

