Pulmonary neuroendocrine cells: physiology, tissue homeostasis and disease
- PMID: 33355253
- PMCID: PMC7774893
- DOI: 10.1242/dmm.046920
Pulmonary neuroendocrine cells: physiology, tissue homeostasis and disease
Abstract
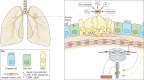
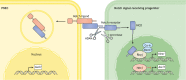
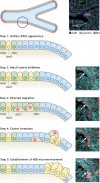
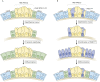
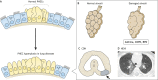
Mammalian lungs have the ability to recognize external environments by sensing different compounds in inhaled air. Pulmonary neuroendocrine cells (PNECs) are rare, multi-functional epithelial cells currently garnering attention as intrapulmonary sensors; PNECs can detect hypoxic conditions through chemoreception. Because PNEC overactivation has been reported in patients suffering from respiratory diseases - such as asthma, chronic obstructive pulmonary disease, bronchopulmonary dysplasia and other congenital diseases - an improved understanding of the fundamental characteristics of PNECs is becoming crucial in pulmonary biology and pathology. During the past decade, murine genetics and disease models revealed the involvement of PNECs in lung ventilation dynamics, mechanosensing and the type 2 immune responses. Single-cell RNA sequencing further unveiled heterogeneous gene expression profiles in the PNEC population and revealed that a small number of PNECs undergo reprogramming during regeneration. Aberrant large clusters of PNECs have been observed in neuroendocrine tumors, including small-cell lung cancer (SCLC). Modern innovation of imaging analyses has enabled the discovery of dynamic migratory behaviors of PNECs during airway development, perhaps relating to SCLC malignancy. This Review summarizes the findings from research on PNECs, along with novel knowledge about their function. In addition, it thoroughly addresses the relevant questions concerning the molecular pathology of pulmonary diseases and related therapeutic approaches.
Keywords: Development; Lung; Neuroendocrine; Regeneration; Respiratory diseases; Vagal nerves.
© 2020. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures

References
-
- Adriaensen D., Timmermans J. P., Brouns I., Berthoud H. R., Neuhuber W. L. and Scheuermann D. W (1998). Pulmonary intraepithelial vagal nodose afferent nerve terminals are confined to neuroepithelial bodies: an anterograde tracing and confocal microscopy study in adult rats. Cell Tissue Res. 293, 395-405. 10.1007/s004410051131 - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

