Myocarditis-associated necrotizing coronary vasculitis: incidence, cause, and outcome
- PMID: 33355356
- PMCID: PMC8088814
- DOI: 10.1093/eurheartj/ehaa973
Myocarditis-associated necrotizing coronary vasculitis: incidence, cause, and outcome
Abstract
Aims: Necrotizing coronary vasculitis (NCV) is a rare entity usually associated to myocarditis which incidence, cause, and response to therapy is unreported.
Methods and results: Among 1916 patients with biopsy-proven myocarditis, 30 had NCV. Endomyocardial samples were retrospectively investigated with immunohistochemistry for toll-like receptor 4 (TLR4) and real-time polymerase chain reaction (PCR) for viral genomes. Serum samples were processed for anti-heart autoantibodies (Abs), IL-1β, IL-6, IL-8, tumour necrosis factor (TNF)-α. Identification of an immunologic pathway (including virus-negativity, TLR4-, and Ab-positivity) was followed by immunosuppression. Myocarditis-NCV cohort was followed for 6 months with 2D-echo and/or cardiac magnetic resonance and compared with 60 Myocarditis patients and 30 controls. Increase in left ventricular ejection fraction ≥10% was classified as response to therapy. Control endomyocardial biopsy followed the end of treatment. Twenty-six Myocarditis-NCV patients presented with heart failure; four with electrical instability. Cause of Myocarditis-NCV included infectious agents (10%) and immune-mediated causes (chest trauma 3%; drug hypersensitivity 7%; hypereosinophilic syndrome 3%; primary autoimmune diseases 33%, idiopathic 44%). Abs were positive in immune-mediated Myocarditis-NCV and virus-negative Myocarditis; Myocarditis-NCV patients with Ab+ presented autoreactivity in vessel walls. Toll-like receptor 4 was overexpressed in immune-mediated forms and poorly detectable in viral. Interleukin-1β was significantly higher in Myocarditis-NCV than Myocarditis, the former presenting 24% in-hospital mortality compared with 1.5% of Myocarditis cohort. Immunosuppression induced improvement of cardiac function in 88% of Myocarditis-NCV and 86% of virus-negative Myocarditis patients.
Conclusion: Necrotizing coronary vasculitis is histologically detectable in 1.5% of Myocarditis. Necrotizing coronary vasculitis includes viral and immune-mediated causes. Intra-hospital mortality is 24%. The immunologic pathway is associated with beneficial response to immunosuppression.
Keywords: Autoimmune disease; Myocarditis; Vasculitis; Viral infection.
© The Author(s) 2021. Published by Oxford University Press on behalf of the European Society of Cardiology.
Figures

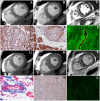
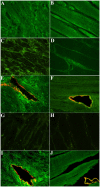
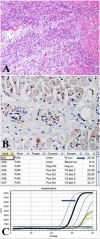
Comment in
-
Myocarditis: endomyocardial biopsy and circulating anti-heart autoantibodies are key to diagnosis and personalized etiology-directed treatment.Eur Heart J. 2021 Apr 21;42(16):1618-1620. doi: 10.1093/eurheartj/ehab024. Eur Heart J. 2021. PMID: 33538808 No abstract available.
References
-
- Schultheiss HP, Kühl U, Cooper LT. The management of myocarditis. Eur Heart J 2011;32:2616–2625. - PubMed
-
- Caforio AL, Calabrese F, Angelini A, Tona F, Vinci A, Bottaro S, Ramondo A, Carturan E, Iliceto S, Thiene G, Daliento L. A prospective study of biopsy-proven myocarditis: prognostic relevance of clinical and aetiopathogenetic features at diagnosis. Eur Heart J 2007;28:1326–1333. - PubMed
-
- Caforio AL, Pankuweit S, Arbustini E, Basso C, Gimeno-Blanes J, Felix SB, Fu M, Heliö T, Heymans S, Jahns R, Klingel K, Linhart A, Maisch B, McKenna W, Mogensen J, Pinto YM, Ristic A, Schultheiss HP, Seggewiss H, Tavazzi L, Thiene G, Yilmaz A, Charron P, Elliott PM; European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J 2013;34:2636–2648, 2648a–2648d. - PubMed
-
- Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2015;16:233–270. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

