PMCA4 gene expression is regulated by the androgen receptor in the mouse testis during spermatogenesis
- PMID: 33355366
- PMCID: PMC7789089
- DOI: 10.3892/mmr.2020.11791
PMCA4 gene expression is regulated by the androgen receptor in the mouse testis during spermatogenesis
Abstract
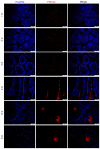
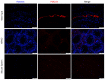
The present study aimed to investigate the expression of ATPase Ca++ transporting plasma membrane 4 (PMCA4) in mouse testis and to determine its role in spermatogenesis. Reverse transcription‑quantitative PCR, western blotting and immunofluorescence were performed to evaluate the expression levels of PMCA4 in mouse testes at various weeks postnatal in wild type mice, and in testes from Sertoli cell‑specific androgen receptor knockout and androgen receptor knockout (ARKO) mice. Luciferase assay, androgen receptor (AR) overexpression and AR antagonist experiments were used to confirm that AR regulated the expression of PMCA4. The results demonstrated that PMCA4 was highly expressed in mouse testes at 3‑8 weeks postnatal. PMCA4 expression levels in ARKO mouse testes were decreased compared with wild type. In addition, activation of AR by testosterone administration resulted in an increase in the activity of the PMCA4 promoter. Cells transfected with an AR‑overexpressing plasmid exhibited increased expression levels of the PMCA4 protein. Finally, the increase in PMCA4 protein levels induced by testosterone was prevented by pre‑treatment with the AR antagonist flutamide. The present results confirmed that PMCA4 was upregulated during mouse testis development and that PMCA4 mRNA and protein expression levels were regulated by androgens and AR. The present findings suggest that PMCA4 may be involved in the regulation of spermatogenesis.
Keywords: ATPase Ca++ transporting plasma membrane 4; spermatogenesis; testis; androgen receptor.
Figures





References
-
- De Gendt K, Swinnen JV, Saunders PT, Schoonjans L, Dewerchin M, Devos A, Tan K, Atanassova N, Claessens F, Lécureuil C, et al. A Sertoli cell-selective knockout of the androgen receptor causes spermatogenic arrest in meiosis. Proc Natl Acad Sci USA. 2004;101:1327–1332. doi: 10.1073/pnas.0308114100. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

