Predicting wet age-related macular degeneration (AMD) using DARC (detecting apoptosing retinal cells) AI (artificial intelligence) technology
- PMID: 33355491
- PMCID: PMC8011474
- DOI: 10.1080/14737159.2020.1865806
Predicting wet age-related macular degeneration (AMD) using DARC (detecting apoptosing retinal cells) AI (artificial intelligence) technology
Abstract
Objectives: To assess a recently described CNN (convolutional neural network) DARC (Detection of Apoptosing Retinal Cells) algorithm in predicting new Subretinal Fluid (SRF) formation in Age-related-Macular-Degeneration (AMD).
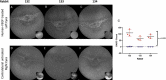
Methods: Anonymized DARC, baseline and serial OCT images (n = 427) from 29 AMD eyes of Phase 2 clinical trial (ISRCTN10751859) were assessed with CNN algorithms, enabling the location of each DARC spot on corresponding OCT slices (n = 20,629). Assessment of DARC in a rabbit model of angiogenesis was performed in parallel.
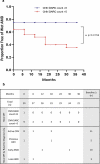
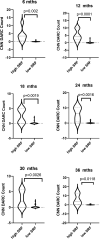
Results: A CNN DARC count >5 at baseline was significantly (p = 0.0156) related to development of new SRF throughout 36 months. Prediction rate of eyes using unique DARC spots overlying new SRF had positive predictive values, sensitivities and specificities >70%, with DARC count significantly (p < 0.005) related to the magnitude of SRF accumulation at all time points. DARC identified earliest stages of angiogenesis in-vivo.
Conclusions: DARC was able to predict new wet-AMD activity. Using only an OCT-CNN definition of new SRF, we demonstrate that DARC can identify early endothelial neovascular activity, as confirmed by rabbit studies. Although larger validation studies are required, this shows the potential of DARC as a biomarker of wet AMD, and potentially saving vision-loss.
Keywords: AMD; CNV; DARC; SRF; angiogenesis; biomarker.
Figures
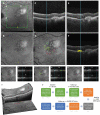
References
-
- Rein DB, Wittenborn JS, Zhang X, et al. Forecasting age-related macular degeneration through the year 2050: the potential impact of new treatments. Arch Ophthalmol. 2009. April;127(4):533–540. . - PubMed
-
- Lindekleiv H, Erke MG.. Projected prevalence of age-related macular degeneration in Scandinavia 2012–2040. Acta Ophthalmol. 2013. June;91(4):307–311. . - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
