Pharmacokinetic interaction study between ligustrazine and valsartan in rats and its potential mechanism
- PMID: 33355495
- PMCID: PMC7759250
- DOI: 10.1080/13880209.2020.1859554
Pharmacokinetic interaction study between ligustrazine and valsartan in rats and its potential mechanism
Abstract
Context: Ligustrazine and valsartan are commonly used drugs in the treatment of cardiac and cardiovascular disease.
Objective: The interaction between ligustrazine and valsartan was studied to investigate the effect of ligustrazine on the pharmacokinetics of valsartan.
Materials and methods: The pharmacokinetics of valsartan (10 mg/kg) was investigated in Sprague-Dawley rats divided into three groups (with the pretreatment of 4 or 10 mg/kg/day ligustrazine for 10 days and without the pretreatment of ligustrazine as control) of six rats each. The in vitro experiments in rat liver microsomes were performed to explore the effect of ligustrazine on the metabolic stability of valsartan.
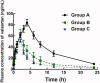
Results: Ligustrazine changed the pharmacokinetic profile of valsartan. In the presence of 4 mg/kg ligustrazine, the AUC(0- t ) (385.37 ± 93.05 versus 851.64 ± 104.26 μg/L*h), t1/2 (5.46 ± 0.93 versus 6.34 ± 1.25 h), and C max (62.64 ± 9.09 versus 83.87 ± 6.15 μg/L) of valsartan was significantly decreased, and the clearance rate was increased from 10.92 ± 1.521 to 25.76 ± 6.24 L/h/kg and similar changes were observed in the group with 10 mg/kg ligustrazine (p < 0.05). The metabolic stability of valsartan was also decreased by ligustrazine as the half-life of valsartan in rat liver microsomes decreased from 37.12 ± 4.06 to 33.48 ± 3.56 min and the intrinsic clearance rate increased from 37.34 ± 3.84 to 41.40 ± 4.32 μL/min/mg protein (p < 0.05).
Discussion and conclusions: Ligustrazine promoted the metabolism of valsartan via activating CYP3A4. The co-administration of ligustrazine and valsartan should be taken into account.
Keywords: CYP3A4; Metabolic stability; drug–drug interaction; metabolism.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures
References
-
- Cai J, Huang Z, Yang G, Cheng K, Ye Q, Ming Y, Zuo X, Zhou P, Yuan H.. 2011. Comparing antihypertensive effect and plasma ciclosporin concentration between amlodipine and valsartan regimens in hypertensive renal transplant patients receiving ciclosporin therapy. Am J Cardiovasc Drugs. 11(6):401–409. - PubMed
-
- Challa VR, Babu PR, Challa SR, Johnson B, Maheswari C.. 2013. Pharmacokinetic interaction study between quercetin and valsartan in rats and in vitro models. Drug Dev Ind Pharm. 39(6):865–872. - PubMed
-
- Cheng ZJ, Vaskonen T, Tikkanen I, Nurminen K, Ruskoaho H, Vapaatalo H, Muller D, Park JK, Luft FC, Mervaala EM.. 2001. Endothelial dysfunction and salt-sensitive hypertension in spontaneously diabetic Goto-Kakizaki rats. Hypertension. 37(2 Pt 2):433–439. - PubMed
-
- Hockenberry B. 1991. Multiple drug therapy in the treatment of essential hypertension. The Nursing Clinics of North America. 26(2):417–436. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources