Cost-Utility Analysis of a Dolutegravir-Based Versus Low-Dose Efavirenz-Based Regimen for the Initial Treatment of HIV-Infected Patients in Cameroon (NAMSAL ANRS 12313 Trial)
- PMID: 33355914
- PMCID: PMC7882571
- DOI: 10.1007/s40273-020-00987-3
Cost-Utility Analysis of a Dolutegravir-Based Versus Low-Dose Efavirenz-Based Regimen for the Initial Treatment of HIV-Infected Patients in Cameroon (NAMSAL ANRS 12313 Trial)
Abstract
Objectives: Evidence comparing the economic and patient values of the World Health Organization's preferred (dolutegravir 50 mg [DTG]-based) and alternative (low-dose [400 mg] efavirenz [EFV400]-based) first-line antiretroviral regimens is limited. We compared patient-reported outcomes (PROs), costs, and the cost-utility of DTG- versus EFV400-based regimens in treatment-naive HIV-1 adults in the randomised NAMSAL ANRS 12313 trial in Yaoundé, Cameroon.
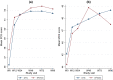
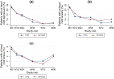
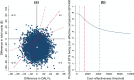
Methods: We used clinical data, PROs, and health resource use data collected in the trial's first 96 weeks (2016-2019). Quality-adjusted life-years (QALYs) were computed using utility scores obtained from the 12-item Short Form (SF-12) generic health scale. Other PROs included perceived symptoms, depression, anxiety, and stress. In the 96-week base-case analysis, we estimated the unadjusted and multivariate-adjusted (1) mean costs (in US$, 2016 values) and QALYs/patient, (2) incremental costs and QALYs/patient, and (3) net health benefit (NHB). Outcomes were extrapolated over 5 and 10 years. Uncertainty was assessed using the cost-effectiveness acceptability curve and scenario and cost-effective price threshold analyses.
Results: In the base-case analysis, the NHB (95% confidence interval) for the DTG-based regimen relative to the EFV400-based regimen was 0.056 (- 0.037 to 0.153), corresponding to an 88% probability of DTG being cost-effective. A 10% decrease in this regimen's price (from $5.2 to $4.7/month) would increase its cost-effectiveness probability to 95%. When extrapolating outcomes over 5 and 10 years, the DTG-based regimen had a 100% probability of being cost-effective for a large range of cost-effectiveness thresholds.
Conclusions: At 2020 antiretroviral drug prices, a DTG-based first-line regimen should be preferred over an EFV400-based regimen in sub-Saharan Africa.
Trial registration: ClinicalTrials.gov Identifier: NCT02777229.
Conflict of interest statement
All authors report no conflict of interest in relation to this study.
Figures

References
-
- World Health Organization. Updated recommendations on first-line and second-line antiretroviral regimens and post-exposure prophylaxis and recommendations on early infant diagnosis of HIV [Internet]. Geneva: World Health Organization; 2018. Report No.: WHO/CDS/HIV/18.51. Available from: http://www.who.int/hiv/pub/guidelines/ARV2018update/en/.
-
- World Health Organization. Statement on DTG—Geneva 18 May 2018 [Internet]. Geneva: World Health Organization; 2018. Available from: https://www.who.int/medicines/publications/drugalerts/Statement_on_DTG_1....
-
- Hill AM, Mitchell N, Hughes S, Pozniak AL. Risks of cardiovascular or central nervous system adverse events and immune reconstitution inflammatory syndrome, for dolutegravir versus other antiretrovirals: meta-analysis of randomized trials. Curr Opin HIV AIDS. 2018;13:102–111. doi: 10.1097/COH.0000000000000445. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

