Sequential CD19 and BCMA-specific CAR T-cell treatment elicits sustained remission of relapsed and/or refractory myeloma
- PMID: 33356013
- PMCID: PMC7877347
- DOI: 10.1002/cam4.3624
Sequential CD19 and BCMA-specific CAR T-cell treatment elicits sustained remission of relapsed and/or refractory myeloma
Abstract
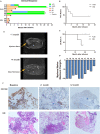
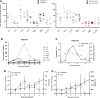
The low rate of durable response against relapsed and/or refractory multiple myeloma (RRMM) in recent studies indicates that chimeric antigen receptor T-cell (CART) treatment is yet to be optimized. This study aims to investigate the safety and efficacy of sequential infusion of CD19-CART and B-cell maturation antigen (BCMA)-CARTs for RRMM with a similar 3 + 3 dose escalation combined with a toxicity sentinel design. We enrolled 10 patients, among whom 7 received autologous infusion and 3 received allogeneic infusion. The median follow-up time was 20 months. The most common grade 3/4 treatment-emergent toxicities were hematological toxicities. Cytokine-release syndrome (CRS) adverse reactions were grade 1/2 in 9 out of 10 subjects. No dose-limited toxicity (DLT) was observed for BCMA-CAR-positive T cells ≤5 × 107 /kg), while two patients with dose-levels of 5-6.5 × 107 /kg experienced DLTs. The overall response rate was 90% (five partial responses and four stringent complete responses). Three out of four patients with stringent complete responses to autologous CART had progression-free survival for over 2 years. The three patients with allogeneic CART experienced disease progression within 2 months. These results evidence the sequential infusion's preliminarily tolerability and efficacy in RRMM, and present a simple and safe design applicable for the establishment of multiple CART therapy.
Keywords: chimeric antigen receptor T (CAR T) cell; dose-escalation; efficacy; multiple myeloma; relapsed and/or refractory; safety.
© 2020 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
All authors declare that they have no conflict of interest. This manuscript involving the research protocol had been approved by the Ethics Committee of the First Affiliated Hospital of Soochow University and the Helsinki Declaration of 1975, as revised in 2000 (5). Informed consent was obtained from all the enrolled patients in the study.
Figures


Similar articles
-
Efficacy and Safety of CAR-T Therapy for Relapse or Refractory Multiple Myeloma: A systematic review and meta-analysis.Int J Med Sci. 2021 Feb 18;18(8):1786-1797. doi: 10.7150/ijms.46811. eCollection 2021. Int J Med Sci. 2021. PMID: 33746596 Free PMC article.
-
A bispecific CAR-T cell therapy targeting BCMA and CD38 in relapsed or refractory multiple myeloma.J Hematol Oncol. 2021 Oct 9;14(1):161. doi: 10.1186/s13045-021-01170-7. J Hematol Oncol. 2021. PMID: 34627333 Free PMC article. Clinical Trial.
-
A combination of humanised anti-CD19 and anti-BCMA CAR T cells in patients with relapsed or refractory multiple myeloma: a single-arm, phase 2 trial.Lancet Haematol. 2019 Oct;6(10):e521-e529. doi: 10.1016/S2352-3026(19)30115-2. Epub 2019 Aug 1. Lancet Haematol. 2019. PMID: 31378662 Clinical Trial.
-
A phase 1 study of a novel fully human BCMA-targeting CAR (CT103A) in patients with relapsed/refractory multiple myeloma.Blood. 2021 May 27;137(21):2890-2901. doi: 10.1182/blood.2020008936. Blood. 2021. PMID: 33512480 Clinical Trial.
-
Efficacy and safety of chimeric antigen receptor T cells targeting BCMA and GPRC5D in relapsed or refractory multiple myeloma.Front Immunol. 2024 Dec 23;15:1466443. doi: 10.3389/fimmu.2024.1466443. eCollection 2024. Front Immunol. 2024. PMID: 39763668 Free PMC article.
Cited by
-
Chimeric Antigen Receptor T-Cell Therapy: What We Expect Soon.Int J Mol Sci. 2022 Nov 1;23(21):13332. doi: 10.3390/ijms232113332. Int J Mol Sci. 2022. PMID: 36362130 Free PMC article. Review.
-
Current approaches to develop "off-the-shelf" chimeric antigen receptor (CAR)-T cells for cancer treatment: a systematic review.Exp Hematol Oncol. 2023 Aug 21;12(1):73. doi: 10.1186/s40164-023-00435-w. Exp Hematol Oncol. 2023. PMID: 37605218 Free PMC article. Review.
-
Clinical efficacy and safety of combined anti-BCMA and anti-CD19 CAR-T cell therapy for relapsed/refractory multiple myeloma: a systematic review and meta-analysis.Front Oncol. 2024 Apr 8;14:1355643. doi: 10.3389/fonc.2024.1355643. eCollection 2024. Front Oncol. 2024. PMID: 38651157 Free PMC article.
-
Current Anti-Myeloma Chimeric Antigen Receptor-T Cells: Novel Targets and Methods.Balkan Med J. 2025 Jul 1;42(4):301-310. doi: 10.4274/balkanmedj.galenos.2025.2025-4-25. Balkan Med J. 2025. PMID: 40619794 Free PMC article. Review.
-
Strategies for Reducing Toxicity and Enhancing Efficacy of Chimeric Antigen Receptor T Cell Therapy in Hematological Malignancies.Int J Mol Sci. 2023 May 23;24(11):9115. doi: 10.3390/ijms24119115. Int J Mol Sci. 2023. PMID: 37298069 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

