Sequential CD19 and BCMA-specific CAR T-cell treatment elicits sustained remission of relapsed and/or refractory myeloma
- PMID: 33356013
- PMCID: PMC7877347
- DOI: 10.1002/cam4.3624
Sequential CD19 and BCMA-specific CAR T-cell treatment elicits sustained remission of relapsed and/or refractory myeloma
Abstract
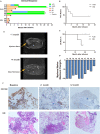
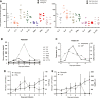
The low rate of durable response against relapsed and/or refractory multiple myeloma (RRMM) in recent studies indicates that chimeric antigen receptor T-cell (CART) treatment is yet to be optimized. This study aims to investigate the safety and efficacy of sequential infusion of CD19-CART and B-cell maturation antigen (BCMA)-CARTs for RRMM with a similar 3 + 3 dose escalation combined with a toxicity sentinel design. We enrolled 10 patients, among whom 7 received autologous infusion and 3 received allogeneic infusion. The median follow-up time was 20 months. The most common grade 3/4 treatment-emergent toxicities were hematological toxicities. Cytokine-release syndrome (CRS) adverse reactions were grade 1/2 in 9 out of 10 subjects. No dose-limited toxicity (DLT) was observed for BCMA-CAR-positive T cells ≤5 × 107 /kg), while two patients with dose-levels of 5-6.5 × 107 /kg experienced DLTs. The overall response rate was 90% (five partial responses and four stringent complete responses). Three out of four patients with stringent complete responses to autologous CART had progression-free survival for over 2 years. The three patients with allogeneic CART experienced disease progression within 2 months. These results evidence the sequential infusion's preliminarily tolerability and efficacy in RRMM, and present a simple and safe design applicable for the establishment of multiple CART therapy.
Keywords: chimeric antigen receptor T (CAR T) cell; dose-escalation; efficacy; multiple myeloma; relapsed and/or refractory; safety.
© 2020 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
All authors declare that they have no conflict of interest. This manuscript involving the research protocol had been approved by the Ethics Committee of the First Affiliated Hospital of Soochow University and the Helsinki Declaration of 1975, as revised in 2000 (5). Informed consent was obtained from all the enrolled patients in the study.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

