A Neutralizing Monoclonal Antibody for Hospitalized Patients with Covid-19
- PMID: 33356051
- PMCID: PMC7781100
- DOI: 10.1056/NEJMoa2033130
A Neutralizing Monoclonal Antibody for Hospitalized Patients with Covid-19
Abstract
Background: LY-CoV555, a neutralizing monoclonal antibody, has been associated with a decrease in viral load and the frequency of hospitalizations or emergency department visits among outpatients with coronavirus disease 2019 (Covid-19). Data are needed on the effect of this antibody in patients who are hospitalized with Covid-19.
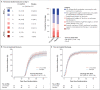
Methods: In this platform trial of therapeutic agents, we randomly assigned hospitalized patients who had Covid-19 without end-organ failure in a 1:1 ratio to receive either LY-CoV555 or matching placebo. In addition, all the patients received high-quality supportive care as background therapy, including the antiviral drug remdesivir and, when indicated, supplemental oxygen and glucocorticoids. LY-CoV555 (at a dose of 7000 mg) or placebo was administered as a single intravenous infusion over a 1-hour period. The primary outcome was a sustained recovery during a 90-day period, as assessed in a time-to-event analysis. An interim futility assessment was performed on the basis of a seven-category ordinal scale for pulmonary function on day 5.
Results: On October 26, 2020, the data and safety monitoring board recommended stopping enrollment for futility after 314 patients (163 in the LY-CoV555 group and 151 in the placebo group) had undergone randomization and infusion. The median interval since the onset of symptoms was 7 days (interquartile range, 5 to 9). At day 5, a total of 81 patients (50%) in the LY-CoV555 group and 81 (54%) in the placebo group were in one of the two most favorable categories of the pulmonary outcome. Across the seven categories, the odds ratio of being in a more favorable category in the LY-CoV555 group than in the placebo group was 0.85 (95% confidence interval [CI], 0.56 to 1.29; P = 0.45). The percentage of patients with the primary safety outcome (a composite of death, serious adverse events, or clinical grade 3 or 4 adverse events through day 5) was similar in the LY-CoV555 group and the placebo group (19% and 14%, respectively; odds ratio, 1.56; 95% CI, 0.78 to 3.10; P = 0.20). The rate ratio for a sustained recovery was 1.06 (95% CI, 0.77 to 1.47).
Conclusions: Monoclonal antibody LY-CoV555, when coadministered with remdesivir, did not demonstrate efficacy among hospitalized patients who had Covid-19 without end-organ failure. (Funded by Operation Warp Speed and others; TICO ClinicalTrials.gov number, NCT04501978.).
Copyright © 2020 Massachusetts Medical Society.
Figures
Comment in
-
Monoclonal Antibody for Patients with Covid-19.N Engl J Med. 2021 Mar 25;384(12):1170-1171. doi: 10.1056/NEJMc2100221. Epub 2021 Feb 3. N Engl J Med. 2021. PMID: 33534967 No abstract available.
References
-
- National Institutes of Health. Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV). 2020. (https://www.nih.gov/research-training/medical-research-initiatives/activ).
-
- Jones BE, Brown-Augsburger PL, Corbett KS, et al. LY-CoV555, a rapidly isolated potent neutralizing antibody, provides protection in a non-human primate model of SARS-CoV-2 infection. October 9, 2020. (https://www.biorxiv.org/content/10.1101/2020.09.30.318972v3). preprint.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
