Chemically Modified Biopolymers for the Formation of Biomedical Hydrogels
- PMID: 33356174
- PMCID: PMC8943712
- DOI: 10.1021/acs.chemrev.0c00923
Chemically Modified Biopolymers for the Formation of Biomedical Hydrogels
Abstract
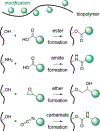
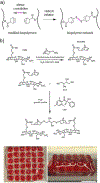
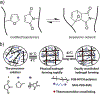
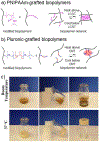
Biopolymers are natural polymers sourced from plants and animals, which include a variety of polysaccharides and polypeptides. The inclusion of biopolymers into biomedical hydrogels is of great interest because of their inherent biochemical and biophysical properties, such as cellular adhesion, degradation, and viscoelasticity. The objective of this Review is to provide a detailed overview of the design and development of biopolymer hydrogels for biomedical applications, with an emphasis on biopolymer chemical modifications and cross-linking methods. First, the fundamentals of biopolymers and chemical conjugation methods to introduce cross-linking groups are described. Cross-linking methods to form biopolymer networks are then discussed in detail, including (i) covalent cross-linking (e.g., free radical chain polymerization, click cross-linking, cross-linking due to oxidation of phenolic groups), (ii) dynamic covalent cross-linking (e.g., Schiff base formation, disulfide formation, reversible Diels-Alder reactions), and (iii) physical cross-linking (e.g., guest-host interactions, hydrogen bonding, metal-ligand coordination, grafted biopolymers). Finally, recent advances in the use of chemically modified biopolymer hydrogels for the biofabrication of tissue scaffolds, therapeutic delivery, tissue adhesives and sealants, as well as the formation of interpenetrating network biopolymer hydrogels, are highlighted.
Figures



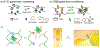

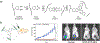

Similar articles
-
Click chemistry-based biopolymeric hydrogels for regenerative medicine.Biomed Mater. 2021 Mar 16;16(2):022003. doi: 10.1088/1748-605X/abc0b3. Biomed Mater. 2021. PMID: 33049725
-
Recent advances in biopolymer-based hydrogels and their potential biomedical applications.Carbohydr Polym. 2024 Jan 1;323:121408. doi: 10.1016/j.carbpol.2023.121408. Epub 2023 Sep 17. Carbohydr Polym. 2024. PMID: 37940291 Review.
-
Composite hydrogels assembled from food-grade biopolymers: Fabrication, properties, and applications.Adv Colloid Interface Sci. 2024 Oct;332:103278. doi: 10.1016/j.cis.2024.103278. Epub 2024 Aug 14. Adv Colloid Interface Sci. 2024. PMID: 39153416 Review.
-
Dynamic protein and polypeptide hydrogels based on Schiff base co-assembly for biomedicine.J Mater Chem B. 2022 May 4;10(17):3173-3198. doi: 10.1039/d2tb00077f. J Mater Chem B. 2022. PMID: 35352081 Review.
-
Bonding interactions and stability assessment of biopolymer material prepared using type III collagen of avian intestine and anionic polysaccharides.J Mater Sci Mater Med. 2011 Jun;22(6):1419-29. doi: 10.1007/s10856-011-4337-0. Epub 2011 May 6. J Mater Sci Mater Med. 2011. PMID: 21547588
Cited by
-
Advanced injectable hydrogels for cartilage tissue engineering.Front Bioeng Biotechnol. 2022 Sep 8;10:954501. doi: 10.3389/fbioe.2022.954501. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 36159703 Free PMC article. Review.
-
In Situ Photo-crosslinkable Hyaluronic Acid/Gelatin Hydrogel for Local Nitric Oxide Delivery.ACS Appl Mater Interfaces. 2023 Oct 25;15(42):48930-48944. doi: 10.1021/acsami.3c10030. Epub 2023 Oct 12. ACS Appl Mater Interfaces. 2023. PMID: 37827196 Free PMC article.
-
Applications of Hydrogels in Osteoarthritis Treatment.Biomedicines. 2024 Apr 22;12(4):923. doi: 10.3390/biomedicines12040923. Biomedicines. 2024. PMID: 38672277 Free PMC article. Review.
-
The Promise and Challenges of Bioprinting in Tissue Engineering.Micromachines (Basel). 2024 Dec 23;15(12):1529. doi: 10.3390/mi15121529. Micromachines (Basel). 2024. PMID: 39770282 Free PMC article.
-
Harnessing the potential of hydrogels for advanced therapeutic applications: current achievements and future directions.Signal Transduct Target Ther. 2024 Jul 1;9(1):166. doi: 10.1038/s41392-024-01852-x. Signal Transduct Target Ther. 2024. PMID: 38945949 Free PMC article. Review.
References
-
- Caló E; Khutoryanskiy VV Biomedical Applications of Hydrogels: A Review of Patents and Commercial Products. Eur. Polym. J 2015, 65, 252–267.
-
- Janouskova O Synthetic Polymer Scaffolds for Soft Tissue Engineering. Physiol. Res 2018, 67. - PubMed
-
- Van Vlierberghe S; Dubruel P; Schacht E Biopolymer-Based Hydrogels as Scaffolds for Tissue Engineering Applications: A Review. Biomacromolecules 2011, 12, 1387–1408. - PubMed
-
- Necas J; Bartosikova L; Brauner P; Kolar J Hyaluronic Acid (Hyaluronan): A Review. Vet. Med. (Praha) 2008, 53, 397–411.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

