Intracellular AIBP (Apolipoprotein A-I Binding Protein) Regulates Oxidized LDL (Low-Density Lipoprotein)-Induced Mitophagy in Macrophages
- PMID: 33356389
- PMCID: PMC8105271
- DOI: 10.1161/ATVBAHA.120.315485
Intracellular AIBP (Apolipoprotein A-I Binding Protein) Regulates Oxidized LDL (Low-Density Lipoprotein)-Induced Mitophagy in Macrophages
Abstract
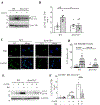
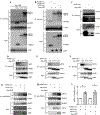
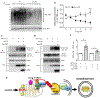
Objective: Atherosclerotic lesions are often characterized by accumulation of OxLDL (oxidized low-density lipoprotein), which is associated with vascular inflammation and lesion vulnerability to rupture. Extracellular AIBP (apolipoprotein A-I binding protein; encoded by APOA1BP gene), when secreted, promotes cholesterol efflux and regulates lipid rafts dynamics, but its role as an intracellular protein in mammalian cells remains unknown. The aim of this work was to determine the function of intracellular AIBP in macrophages exposed to OxLDL and in atherosclerotic lesions. Approach and Results: Using a novel monoclonal antibody against human and mouse AIBP, which are highly homologous, we demonstrated robust AIBP expression in human and mouse atherosclerotic lesions. We observed significantly reduced autophagy in bone marrow-derived macrophages, isolated from Apoa1bp-/- compared with wild-type mice, which were exposed to OxLDL. In atherosclerotic lesions from Apoa1bp-/- mice subjected to Ldlr knockdown and fed a Western diet, autophagy was reduced, whereas apoptosis was increased, when compared with that in wild-type mice. AIBP expression was necessary for efficient control of reactive oxygen species and cell death and for mitochondria quality control in macrophages exposed to OxLDL. Mitochondria-localized AIBP, via its N-terminal domain, associated with E3 ubiquitin-protein ligase PARK2 (Parkin), MFN (mitofusin)1, and MFN2, but not BNIP3 (Bcl2/adenovirus E1B 19-kDa-interacting protein-3), and regulated ubiquitination of MFN1 and MFN2, key components of mitophagy.
Conclusions: These data suggest that intracellular AIBP is a new regulator of autophagy in macrophages. Mitochondria-localized AIBP augments mitophagy and participates in mitochondria quality control, protecting macrophages against cell death in the context of atherosclerosis.
Keywords: atherosclerosis; autophagy; inflammation; macrophages; mitophagy.
Conflict of interest statement
Disclosures
YIM is a scientific co-founder of Raft Pharmaceuticals LLC. ST is a consultant to Boston Heart Diagnostics and is a co-inventor and receives royalties from patents owned by UCSD on oxidation-specific antibodies and of biomarkers related to oxidized lipoproteins, has a dual appointment at UCSD and Ionis Pharmaceuticals, and is a co-founder and has an equity interest in Oxitope, Inc and Kleanthi Diagnostics, LLC. Although these relationships have been identified for conflict of interest management based on the overall scope of the project and its potential benefit to Oxitope and Kleanthi Diagnostics LLC, the research findings included in this particular publication may not necessarily relate to the interests of Oxitope, Inc and Kleanthi Diagnostics, LLC. The terms of this arrangement have been reviewed and approved by the University of California, San Diego in accordance with its conflict of interest policies. Other authors declare that they have no competing interests.
Figures



References
-
- Hansson GK, Libby P The immune response in atherosclerosis: A double-edged sword. Nat Rev Immunol. 2006;6:508–519 - PubMed
-
- Miller YI, Choi SH, Wiesner P, Fang L, Harkewicz R, Hartvigsen K, Boullier A, Gonen A, Diehl CJ, Que X, Montano E, Shaw PX, Tsimikas S, Binder CJ, Witztum JL. Oxidation-specific epitopes are danger-associated molecular patterns recognized by pattern recognition receptors of innate immunity. Circ Res. 2011;108:235–248 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

