Defining the Optimal Total Number of Chemotherapy Courses in Younger Patients With Acute Myeloid Leukemia: A Comparison of Three Versus Four Courses
- PMID: 33356418
- PMCID: PMC8177881
- DOI: 10.1200/JCO.20.01170
Defining the Optimal Total Number of Chemotherapy Courses in Younger Patients With Acute Myeloid Leukemia: A Comparison of Three Versus Four Courses
Abstract
Purpose: The optimum number of treatment courses for younger patients with acute myeloid leukemia (AML) is uncertain. The United Kingdom National Cancer Research Institute AML17 trial randomly assigned patients who were not high risk to a total of three versus four courses.
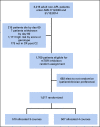
Patients and methods: Patients received two induction courses based on daunorubicin and cytarabine (Ara-C), usually with gemtuzumab ozogamicin. Following remission, 1,017 patients were randomly assigned to a third course, MACE (amsacrine, Ara-C, and etoposide), plus a fourth course of MidAc (mitoxantrone and Ara-C) and following an amendment to one or two courses of high-dose Ara-C. Primary end points were cumulative incidence of relapse (CIR), relapse-free survival (RFS), and overall survival (OS). Outcomes were correlated with patient characteristics, mutations, cytogenetics, induction treatments, and measurable residual disease (MRD) postinduction.
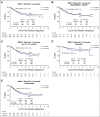
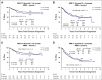
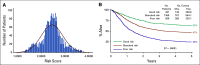
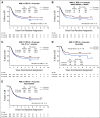
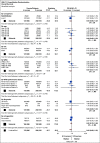
Results: In logrank analyses, CIR and RFS at 5 years were improved in recipients of four courses (50% v 58%: hazard ratio [HR] 0.81 [0.69-0.97], P = .02 and 43% v 36%: HR 0.83 [0.71-0.98], P = .03, respectively). While OS was not significantly better (63% v 57%: HR 0.84 [0.69-1.03], P = .09), the noninferiority of three courses to four courses was not established. The impact on relapse was only significant when the fourth course was Ara-C. In exploratory analyses, although MRD impacted survival, a fourth course had no effect in either MRD-positive or MRD-negative patients. A fourth course was beneficial in patients who lacked a mutation of FLT3 or NPM1, had < 3 mutations in other genes, or had a presenting WBC of < 10 × 109 L-1.
Conclusion: Although a fourth course of high-dose Ara-C reduced CIR and improved RFS, it did not result in a significant OS benefit. Subsets including those with favorable cytogenetics, those lacking a mutation of FLT3 or NPM1, or those with < 3 other mutations may derive survival benefit.
Figures




References
-
- Bishop JF Matthews JP Young GA, et al. : A randomized study of high-dose cytarabine in induction in acute myeloid leukemia. Blood 87:1710-1717, 1996 - PubMed
-
- Holowiecki J Grosicki S Giebel S, et al. : Cladribine, but not fludarabine, added to daunorubicin and cytarabine during induction prolongs survival of patients with acute myeloid leukemia: A multicenter, randomized phase III study. J Clin Oncol 30:2441-2448, 2012 - PubMed
-
- Lee JH Joo YD Kim H, et al. : A randomized trial comparing standard versus high-dose daunorubicin induction in patients with acute myeloid leukemia. Blood 118:3832-3841, 2011 - PubMed
-
- Burnett AK Hills RK Wheatley K, et al. : A sensitive risk score for directing treatment in younger patients with AML. Blood 108:10a, 2006.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

