Paroxysmal nocturnal hemoglobinuria: current treatments and unmet needs
- PMID: 33356783
- PMCID: PMC10410676
- DOI: 10.18553/jmcp.2020.26.12-b.s14
Paroxysmal nocturnal hemoglobinuria: current treatments and unmet needs
Abstract
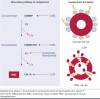
The current standard of care for paroxysmal nocturnal hemoglobinuria (PNH) are the C5 inhibitors eculizumab and ravulizumab, both monoclonal antibodies designed to target the complement protein C5, thereby preventing its cleavage and the formation of the terminal attack complex. C5 inhibitors have yielded substantial improvements in the treatment of PNH and changed the mortality and morbidity, as well as health-related quality of life of patients with the disease. These treatments target underlying intravascular hemolysis; however, they do not address extravascular hemolysis, resulting in incomplete response and remaining symptoms in some patients. Therefore, despite treatment with a C5 inhibitor, some patients still experience anemia with associated fatigue, transfusion needs, and impaired health-related quality of life. DISCLOSURES: This research was developed under a research contract between RTI Health Solutions and Apellis Pharmaceuticals and was funded by Apellis Pharmaceuticals. Bektas, Copley-Merriman, and Khan are employees of RTI Health Solutions. Sarda is an employee of Apellis Pharmaceuticals. Shammo consults for Apellis Pharmaceuticals.
Conflict of interest statement
This research was developed under a research contract between RTI Health Solutions and Apellis Pharmaceuticals and was funded by Apellis Pharmaceuticals. Bektas, Copley-Merriman, and Khan are employees of RTI Health Solutions. Sarda is an employee of Apellis Pharmaceuticals. Shammo consults for Apellis Pharmaceuticals.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous