Inverse regulation of claudin-2 and -7 expression by p53 and hepatocyte nuclear factor 4α in colonic MCE301 cells
- PMID: 33356822
- PMCID: PMC7849757
- DOI: 10.1080/21688370.2020.1860409
Inverse regulation of claudin-2 and -7 expression by p53 and hepatocyte nuclear factor 4α in colonic MCE301 cells
Abstract
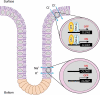
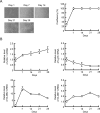
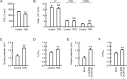
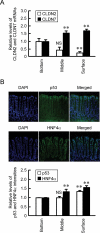
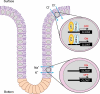
Colonic epithelial cells move up along the crypt villus axis and are differentiated into absorptive or secretory cells. Claudin-7 (CLDN7), a tight junctional protein, is mainly located at the surface of crypt, whereas CLDN2 is located at the bottom. However, the expression mechanism and function of these CLDNs are not fully understood. The expression levels of CLDN2 and CLDN7 were altered depending on the culture days in MCE301 cells derived from mouse colon. The nuclear levels of transcriptional factors p53 and hepatocyte nuclear factor 4α (HNF4α) at day 21 were higher than those at day 7. Tenovin-1 (TEN), a p53 activator, increased the nuclear levels of p53 and HNF4α. The mRNA level and promoter activity of CLDN7 were increased by TEN, whereas those of CLDN2 were decreased. The changes of CLDNs expression were inhibited by p53 and HNF4α siRNAs. The association between p53 and HNF4α was elevated by TEN. In addition, the binding of p53 and HNF4α to the promoter region of CLDN2 and CLDN7 was enhanced by TEN. Transepithelial electrical resistance was decreased by TEN, but paracellular fluxes of lucifer yellow and dextran were not. In the Ussing chamber assay, TEN increased dilution potential and the ratio of permeability of Cl- to Na+. Both p53 and HNF4α were highly expressed at the surface of mouse colon crypt. We suggest that p53 and HNF4α alter the paracellular permeability of Cl- to Na+ mediated by the inverse regulation of CLDN2 and CLDN7 expression in the colon.
Keywords: Colon; HNF4Α; claudin; p53.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
