Optimizing In Vitro Osteogenesis in Canine Autologous and Induced Pluripotent Stem Cell-Derived Mesenchymal Stromal Cells with Dexamethasone and BMP-2
- PMID: 33356875
- PMCID: PMC7891305
- DOI: 10.1089/scd.2020.0144
Optimizing In Vitro Osteogenesis in Canine Autologous and Induced Pluripotent Stem Cell-Derived Mesenchymal Stromal Cells with Dexamethasone and BMP-2
Abstract
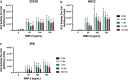
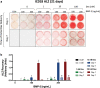
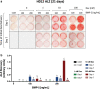
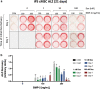
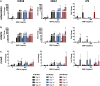
A growing body of work suggests that canine mesenchymal stromal cells (cMSCs) require additional agonists such as bone morphogenic protein-2 (BMP-2) for consistent in vitro osteogenic differentiation. BMP-2 is costly and may challenge the translational relevance of the canine model. Dexamethasone enhances osteogenic differentiation of human MSCs (hMSCs) and is widely utilized in osteogenic protocols. The aim of this study was to determine the effect of BMP-2 and dexamethasone on early- and late-stage osteogenesis of autologous and induced pluripotent stem cell (iPS)-derived cMSCs. Two preparations of marrow-derived cMSCs were selected to represent exceptionally or marginally osteogenic autologous cMSCs. iPS-derived cMSCs were generated from canine fibroblasts. All preparations were evaluated using alkaline phosphatase (ALP) activity, Alizarin Red staining of osteogenic monolayers, and quantitative polymerase chain reaction. Data were reported as mean ± standard deviation and compared using one- or two-way analysis of variance and Tukey or Sidak post hoc tests. Significance was established at P < 0.05. In early-stage assays, dexamethasone decreased ALP activity for all cMSCs in the presence of BMP-2. In late-stage assays, inclusion of dexamethasone and BMP-2 at Day 1 of culture produced robust monolayer mineralization for autologous cMSCs. Delivering 100 nM dexamethasone at Day 1 improved mineralization and reduced the BMP-2 concentrations required to achieve mineralization of the marginal cMSCs. For iPS-cMSCs, dexamethasone was inhibitory to both ALP activity and monolayer mineralization. There was increased expression of osteocalcin and osterix with BMP-2 in autologous cMSCs but a more modest expression occurred in iPS cMSCs. While autologous and iPS-derived cMSCs respond similarly in early-stage osteogenic assays, they exhibit unique responses to dexamethasone and BMP-2 in late-stage mineralization assays. This study demonstrates that dexamethasone and BMP-2 can be titrated in a time- and concentration-dependent manner to enhance osteogenesis of autologous cMSC preparations. These results will prove useful for investigators performing translational studies with cMSCs while providing insight into iPS-derived cMSC osteogenesis.
Keywords: MSCs; bone morphogenic protein-2; canine; induced pluripotent cells; mesenchymal stromal cells; osteogenic differentiation.
Conflict of interest statement
No competing financial interests exist.
Figures
Similar articles
-
In-vitro characterization of canine multipotent stromal cells isolated from synovium, bone marrow, and adipose tissue: a donor-matched comparative study.Stem Cell Res Ther. 2017 Oct 3;8(1):218. doi: 10.1186/s13287-017-0639-6. Stem Cell Res Ther. 2017. PMID: 28974260 Free PMC article.
-
Conditioned Medium Enhances Osteogenic Differentiation of Induced Pluripotent Stem Cell-Derived Mesenchymal Stem Cells.Tissue Eng Regen Med. 2019 Jan 29;16(2):141-150. doi: 10.1007/s13770-018-0173-3. eCollection 2019 Apr. Tissue Eng Regen Med. 2019. PMID: 30989041 Free PMC article.
-
Optimizing the osteogenic differentiation of human mesenchymal stromal cells by the synergistic action of growth factors.J Craniomaxillofac Surg. 2014 Dec;42(8):2002-9. doi: 10.1016/j.jcms.2014.09.006. Epub 2014 Nov 6. J Craniomaxillofac Surg. 2014. PMID: 25458345
-
Evaluating Bioassays for the Determination of Simvastatin's Osteogenic Activity: A Systematic Review.J Funct Biomater. 2025 Feb 11;16(2):61. doi: 10.3390/jfb16020061. J Funct Biomater. 2025. PMID: 39997596 Free PMC article. Review.
-
Why the impact of mechanical stimuli on stem cells remains a challenge.Cell Mol Life Sci. 2018 Sep;75(18):3297-3312. doi: 10.1007/s00018-018-2830-z. Epub 2018 May 4. Cell Mol Life Sci. 2018. PMID: 29728714 Free PMC article. Review.
Cited by
-
Opening the "Black Box" Underlying Barriers to the Use of Canine Induced Pluripotent Stem Cells: A Narrative Review.Stem Cells Dev. 2023 Jun;32(11-12):271-291. doi: 10.1089/scd.2022.0300. Epub 2023 Apr 17. Stem Cells Dev. 2023. PMID: 36884307 Free PMC article. Review.
-
Shape Memory Polymer Scaffolds-Utility for In Vitro Osteogenesis of Canine Multipotent Stromal Cells.J Biomed Mater Res B Appl Biomater. 2024 Dec;112(12):e35503. doi: 10.1002/jbm.b.35503. J Biomed Mater Res B Appl Biomater. 2024. PMID: 39587932 Free PMC article.
-
Canine Mesenchymal Stromal Cell-Mediated Bone Regeneration is Enhanced in the Presence of Sub-Therapeutic Concentrations of BMP-2 in a Murine Calvarial Defect Model.Front Bioeng Biotechnol. 2021 Nov 2;9:764703. doi: 10.3389/fbioe.2021.764703. eCollection 2021. Front Bioeng Biotechnol. 2021. PMID: 34796168 Free PMC article.
-
Recent advances on small molecules in osteogenic differentiation of stem cells and the underlying signaling pathways.Stem Cell Res Ther. 2022 Nov 12;13(1):518. doi: 10.1186/s13287-022-03204-4. Stem Cell Res Ther. 2022. PMID: 36371202 Free PMC article. Review.
-
A three-dimensional (3D), serum-free, Collagen Type I system for chondrogenesis of canine bone marrow-derived multipotent stromal cells (cMSCs).PLoS One. 2022 Jun 9;17(6):e0269571. doi: 10.1371/journal.pone.0269571. eCollection 2022. PLoS One. 2022. PMID: 35679245 Free PMC article.
References
-
- Marcacci M, Kon E, Moukhachev V, Lavroukov A, Kutepov S, Quarto R, Mastrogiacomo M and Cancedda R (2007). Stem cells associated with macroporous bioceramics for long bone repair: 6- to 7-year outcome of a pilot clinical study. Tissue Eng 13:947–955 - PubMed
-
- Viateau V, Logeart-Avramoglou D, Guillemin G and Petite H (2008). Animal models for bone tissue engineering purposes. In: Sourcebook of Models for Biomedical Research. Conn PM, ed. Humana Press, Totowa, NJ, pp 725–736
-
- Reichert JC, Saifzadeh S, Wullschleger ME, Epari DR, Schütz MA, Duda GN, Schell H, van Griensven M, Redl H and Hutmacher DW (2009). The challenge of establishing preclinical models for segmental bone defect research. Biomaterials 30:2149–2163 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

