Awake Prone Positioning Strategy for Nonintubated Hypoxic Patients with COVID-19: A Pilot Trial with Embedded Implementation Evaluation
- PMID: 33356977
- PMCID: PMC8513648
- DOI: 10.1513/AnnalsATS.202009-1164OC
Awake Prone Positioning Strategy for Nonintubated Hypoxic Patients with COVID-19: A Pilot Trial with Embedded Implementation Evaluation
Abstract
Rationale: Prone positioning is an appealing therapeutic strategy for nonintubated hypoxic patients with coronavirus disease (COVID-19), but its effectiveness remains to be established in randomized controlled trials. Objectives: To identify contextual factors relevant to the conduct of a definitive clinical trial evaluating a prone positioning strategy for nonintubated hypoxic patients with COVID-19. Methods: We conducted a cluster randomized pilot trial at a quaternary care teaching hospital. Five inpatient medical service teams were randomly allocated to two treatment arms: 1) usual care (UC), consisting of current, standard management of hypoxia and COVID-19; or 2) the Awake Prone Positioning Strategy (APPS) plus UC. Included patients had positive severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) testing or suspected COVID-19 pneumonia and oxygen saturation less than 93% or new oxygen requirement of 3 L per minute or greater and no contraindications to prone positioning. Oxygenation measures were collected within 48 hours of eligibility and included nadir oxygen saturation to fraction of inspired oxygen (S/F) ratio and time spent with S/F ratio less than 315. Concurrently, we conducted an embedded implementation evaluation using semistructured interviews with clinician and patient participants to determine contextual factors relevant to the successful conduct of a future clinical trial. The primary outcomes were drawn from an implementation science framework including acceptability, adoption, appropriateness, effectiveness, equity, feasibility, fidelity, and penetration. Results: Forty patients were included in the cluster randomized trial. Patients in the UC group (n = 13) had a median nadir S/F ratio over the 48-hour study period of 216 (95% confidence interval [95% CI], 95-303) versus 253 (95% CI, 197-267) in the APPS group (n = 27). Patients in the UC group spent 42 hours (95% CI, 13-47) of the 48-hour study period with an S/F ratio below 315 versus 20 hours (95% CI, 6-39) for patients in the APPS group. Mixed-methods analyses uncovered several barriers relevant to the conduct of a successful definitive randomized controlled trial, including low adherence to prone positioning, large differences between physician-recommended and patient-tolerated prone durations, and diffusion of prone positioning into usual care. Conclusions: A definitive trial evaluating the effect of prone positioning in nonintubated patients with COVID-19 is warranted, but several barriers must be addressed to ensure that the results of such a trial are informative and readily translated into practice.
Keywords: COVID-19; hypoxia; implementation science; prone positioning.
Figures
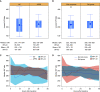
References
-
- Abroug F, Ouanes-Besbes L, Elatrous S, Brochard L. The effect of prone positioning in acute respiratory distress syndrome or acute lung injury: a meta-analysis: areas of uncertainty and recommendations for research. Intensive Care Med. 2008;34:1002–1011. - PubMed
-
- Sud S, Friedrich JO, Taccone P, Polli F, Adhikari NK, Latini R, et al. Prone ventilation reduces mortality in patients with acute respiratory failure and severe hypoxemia: systematic review and meta-analysis. Intensive Care Med. 2010;36:585–599. - PubMed
-
- Gattinoni L, Carlesso E, Taccone P, Polli F, Guérin C, Mancebo J. Prone positioning improves survival in severe ARDS: a pathophysiologic review and individual patient meta-analysis. Minerva Anestesiol. 2010;76:448–454. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

