LRP1 regulates food intake and energy balance in GABAergic neurons independently of leptin action
- PMID: 33356995
- PMCID: PMC8260358
- DOI: 10.1152/ajpendo.00399.2020
LRP1 regulates food intake and energy balance in GABAergic neurons independently of leptin action
Abstract
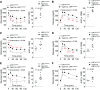
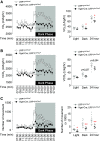
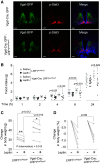
Low-density lipoprotein receptor-related protein 1 (LRP1) is a member of LDL receptor family that plays a key role in systemic glucose and lipid homeostasis. LRP1 also regulates energy balance in the hypothalamus by mediating leptin's anorexigenic action, although the underlying neurocircuitry involved is still unclear. Because GABAergic neurons are a major mediator of hypothalamic leptin action, we studied the role of GABAergic LRP1 in energy balance and leptin action using mice lacking LRP1 in Vgat- or AgRP-expressing neurons (Vgat-Cre; LRP1loxP/loxP or AgRP-Cre; LRP1loxP/loxP). Here, we show that LRP1 deficiency in GABAergic neurons results in severe obesity in male and female mice fed a normal-chow diet. This effect is most likely due to increased food intake and decreased energy expenditure and locomotor activity. Increased adiposity in GABAergic neuron-specific LRP1-deficient mice is accompanied by hyperleptinemia and hyperinsulinemia. Insulin resistance and glucose intolerance in these mice are occurred without change in body weight. Importantly, LRP1 in GABAergic neurons is not required for leptin action, as evidenced by normal leptin's anorexigenic action and leptin-induced hypothalamic Stat3 phosphorylation. In contrast, LRP1 deficiency in AgRP neurons has no effect on adiposity and caloric intake. In conclusion, our data identify GABAergic neurons as a key neurocircuitry that underpins LRP1-dependent regulation of systemic energy balance and body-weight homeostasis. We further find that the GABAergic LRP1 signaling pathway modulates food intake and energy expenditure independently of leptin signaling and AgRP neurons.
Keywords: AgRP; GABAergic; LRP1; Stat3; food intake; leptin.
Figures



Similar articles
-
ROCK1 in AgRP neurons regulates energy expenditure and locomotor activity in male mice.Endocrinology. 2013 Oct;154(10):3660-70. doi: 10.1210/en.2013-1343. Epub 2013 Jul 24. Endocrinology. 2013. PMID: 23885017 Free PMC article.
-
LRP1 in GABAergic neurons is a key link between obesity and memory function.Mol Metab. 2024 Jun;84:101941. doi: 10.1016/j.molmet.2024.101941. Epub 2024 Apr 16. Mol Metab. 2024. PMID: 38636794 Free PMC article.
-
Genetic identification of leptin neural circuits in energy and glucose homeostases.Nature. 2018 Apr;556(7702):505-509. doi: 10.1038/s41586-018-0049-7. Epub 2018 Apr 18. Nature. 2018. PMID: 29670283 Free PMC article.
-
Genetic dissection of neuronal pathways controlling energy homeostasis.Obesity (Silver Spring). 2006 Aug;14 Suppl 5:222S-227S. doi: 10.1038/oby.2006.313. Obesity (Silver Spring). 2006. PMID: 17021371 Review.
-
Leptin and insulin pathways in POMC and AgRP neurons that modulate energy balance and glucose homeostasis.EMBO Rep. 2012 Dec;13(12):1079-86. doi: 10.1038/embor.2012.174. Epub 2012 Nov 13. EMBO Rep. 2012. PMID: 23146889 Free PMC article. Review.
Cited by
-
Leptin hormone and its effectiveness in reproduction, metabolism, immunity, diabetes, hopes and ambitions.J Med Life. 2021 Sep-Oct;14(5):600-605. doi: 10.25122/jml-2021-0153. J Med Life. 2021. PMID: 35027962 Free PMC article. Review.
-
CLK2 in GABAergic neurons is critical in regulating energy balance and anxiety-like behavior in a gender-specific fashion.Front Endocrinol (Lausanne). 2023 Aug 10;14:1172835. doi: 10.3389/fendo.2023.1172835. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37635967 Free PMC article.
-
The Integrated Bioinformatic Approach Reveals the Prognostic Significance of LRP1 Expression in Ovarian Cancer.Int J Mol Sci. 2024 Jul 22;25(14):7996. doi: 10.3390/ijms25147996. Int J Mol Sci. 2024. PMID: 39063239 Free PMC article.
References
-
- Hales C, Carroll MD, Fryar CD, Ogden CL. Prevalence of Obesity and Severe Obesity Among Adults: United States, 2017–2018. Hyattsville, MD: National Center for Health Statistics, 2020. - PubMed
-
- Global BMI Mortality Collaboration, Di Angelantonio E, Bhupathiraju SN, Wormser D, Gao P, Kaptoge S, , et al. . Body-mass index and all-cause mortality: individual-participant-data meta-analysis of 239 prospective studies in four continents. Lancet 388: 776–786, 2016. doi:10.1016/S0140-6736(16)30175-1. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

