Comparative analysis of key immune protection factors in H9N2 avian influenza viruses infected and immunized specific pathogen-free chicken
- PMID: 33357705
- PMCID: PMC7772655
- DOI: 10.1016/j.psj.2020.09.080
Comparative analysis of key immune protection factors in H9N2 avian influenza viruses infected and immunized specific pathogen-free chicken
Abstract
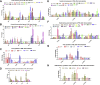
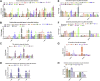
H9N2 avian influenza viruses (AIV) continue to circulate in vaccinated chicken flocks in China, which prompted us to investigate the differential immune protection factors induced by H9N2 AIV infection and immunization for analyzing the reason of protection deficiency of H9N2 AIV inactivated vaccine. In this study, we firstly explored virus-induced optimal immune responses in chicken after H9N2 AIV infection. And, we found that H9N2 hemagglutination inhibition (HI) antibody level, antiviral interferon-stimulated genes including 2',5'-oligoadenylate synthetase-like and myxovirus resistance 1, CD8+ T cell response in peripheral blood lymphocytes (PBL) accompanied by the cytotoxicity-associated genes, including poly (ADP-ribose) polymerase and IFN-r play important roles in defending against H9N2 infection. Besides, we observed that vaccine immunization triggered the similar H9N2 HI antibody level as viral infection, the increase of CD4+ T cell percentage instead of CD8+ T cell percentage in PBL. Moreover, we further made a comparative analysis of immune-related gene expression profile in PBL and lung after H9N2 AIV infection and immunization, respectively. The results showed that vaccine immunization contributed to the up-regulation of Th2 cytokine. But the deficiency of cytotoxicity-associated genes induced by H9N2 AIV inactivated vaccine may be the potential key reason of protection deficiency. These findings provide evidence and direction for developing effective H9N2 AIV vaccines.
Keywords: H9N2 AIV infection; T cell; chicken; immune-related gene; vaccine immunization.
Copyright © 2020. Published by Elsevier Inc.
Figures


References
-
- Edwards S. OIE laboratory standards for avian influenza. Dev. Biol. (Basel) 2006;124:159–162. - PubMed
-
- Fu L., Wang X., Zhai J., Qi W., Jing L., Ge Y., Gao X., Liu C., Lv X., Zheng S. Changes in apoptosis, proliferation and T lymphocyte subtype on thymic cells of SPF chickens infected with reticuloendotheliosis virus. Mol. Immunol. 2019;111:87–94. - PubMed
-
- Li Y., Liu M., Sun Q., Zhang H., Zhang H., Jiang S., Liu S., Huang Y. Genotypic evolution and epidemiological characteristics of H9N2 influenza virus in Shandong Province, China. Poult. Sci. 2019;98:3488–3495. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

