COVID-19 vaccines: The status and perspectives in delivery points of view
- PMID: 33359141
- PMCID: PMC7759095
- DOI: 10.1016/j.addr.2020.12.011
COVID-19 vaccines: The status and perspectives in delivery points of view
Abstract
Due to the high prevalence and long incubation periods often without symptoms, the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) has infected millions of individuals globally, causing the coronavirus disease 2019 (COVID-19) pandemic. Even with the recent approval of the anti-viral drug, remdesivir, and Emergency Use Authorization of monoclonal antibodies against S protein, bamlanivimab and casirimab/imdevimab, efficient and safe COVID-19 vaccines are still desperately demanded not only to prevent its spread but also to restore social and economic activities via generating mass immunization. Recent Emergency Use Authorization of Pfizer and BioNTech's mRNA vaccine may provide a pathway forward, but monitoring of long-term immunity is still required, and diverse candidates are still under development. As the knowledge of SARS-CoV-2 pathogenesis and interactions with the immune system continues to evolve, a variety of drug candidates are under investigation and in clinical trials. Potential vaccines and therapeutics against COVID-19 include repurposed drugs, monoclonal antibodies, antiviral and antigenic proteins, peptides, and genetically engineered viruses. This paper reviews the virology and immunology of SARS-CoV-2, alternative therapies for COVID-19 to vaccination, principles and design considerations in COVID-19 vaccine development, and the promises and roles of vaccine carriers in addressing the unique immunopathological challenges presented by the disease.
Keywords: Coronavirus disease 2019 (COVID-19); Immune response; Neutralizing antibodies; Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2); Vaccine delivery.
Copyright © 2020 Elsevier B.V. All rights reserved.
Figures
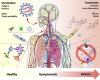
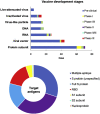






References
-
- Liu D.X., Liang J.Q., Fung T.S. Reference Module in Life Sciences. 2020. Human coronavirus-229E,-OC43,-NL63, and-HKU1. B978-0-12-809633-8.21501-X.
-
- Fani M., Teimoori A., Ghafari S. Comparison of the COVID-2019 (SARS-CoV-2) pathogenesis with SARS-CoV and MERS-CoV infections. Futur. Virol. 2020 doi: 10.2217/fvl-2020-0050. - DOI
-
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

