Isoforms of MUC16 activate oncogenic signaling through EGF receptors to enhance the progression of pancreatic cancer
- PMID: 33359791
- PMCID: PMC8058431
- DOI: 10.1016/j.ymthe.2020.12.029
Isoforms of MUC16 activate oncogenic signaling through EGF receptors to enhance the progression of pancreatic cancer
Abstract
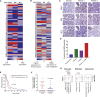
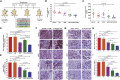
Aberrant expression of CA125/MUC16 is associated with pancreatic ductal adenocarcinoma (PDAC) progression and metastasis. However, knowledge of the contribution of MUC16 to pancreatic tumorigenesis is limited. Here, we show that MUC16 expression is associated with disease progression, basal-like and squamous tumor subtypes, increased tumor metastasis, and short-term survival of PDAC patients. MUC16 enhanced tumor malignancy through the activation of AKT and GSK3β oncogenic signaling pathways. Activation of these oncogenic signaling pathways resulted in part from increased interactions between MUC16 and epidermal growth factor (EGF)-type receptors, which were enhanced for aberrant glycoforms of MUC16. Treatment of PDAC cells with monoclonal antibody (mAb) AR9.6 significantly reduced MUC16-induced oncogenic signaling. mAb AR9.6 binds to a unique conformational epitope on MUC16, which is influenced by O-glycosylation. Additionally, treatment of PDAC tumor-bearing mice with either mAb AR9.6 alone or in combination with gemcitabine significantly reduced tumor growth and metastasis. We conclude that the aberrant expression of MUC16 enhances PDAC progression to an aggressive phenotype by modulating oncogenic signaling through ErbB receptors. Anti-MUC16 mAb AR9.6 blocks oncogenic activities and tumor growth and could be a novel immunotherapeutic agent against MUC16-mediated PDAC tumor malignancy.
Keywords: COSMC; MUC16; Sialyl-Tn; Tn; mAb AR9.6; pancreatic ductal adenocarcinoma.
Copyright © 2020 The American Society of Gene and Cell Therapy. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests M.A.H. and P.R. have an equity interest in OncoCare Therapeutics. R.M. is employed by Quest PharmaTech and has an equity interest in this company. All other authors declare no competing interests.
Figures






References
-
- Marcos-Silva L., Narimatsu Y., Halim A., Campos D., Yang Z., Tarp M.A., Pereira P.J.B., Mandel U., Bennett E.P., Vakhrushev S.Y. Characterization of binding epitopes of CA125 monoclonal antibodies. J. Proteome Res. 2014;13:3349–3359. - PubMed
-
- Liang C., Qin Y., Zhang B., Ji S., Shi S., Xu W., Liu J., Xiang J., Liang D., Hu Q. Oncogenic KRAS Targets MUC16/CA125 in Pancreatic Ductal Adenocarcinoma. Mol. Cancer Res. 2017;15:201–212. - PubMed
-
- O’Brien T.J., Beard J.B., Underwood L.J., Dennis R.A., Santin A.D., York L. The CA 125 gene: an extracellular superstructure dominated by repeat sequences. Tumour Biol. 2001;22:348–366. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

