Polymeric nanoparticle vaccines to combat emerging and pandemic threats
- PMID: 33360074
- PMCID: PMC7834201
- DOI: 10.1016/j.biomaterials.2020.120597
Polymeric nanoparticle vaccines to combat emerging and pandemic threats
Abstract
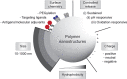
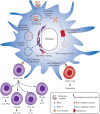
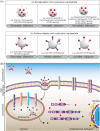
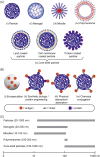
Subunit vaccines are more advantageous than live attenuated vaccines in terms of safety and scale-up manufacture. However, this often comes as a trade-off to their efficacy. Over the years, polymeric nanoparticles have been developed to improve vaccine potency, by engineering their physicochemical properties to incorporate multiple immunological cues to mimic pathogenic microbes and viruses. This review covers recent advances in polymeric nanostructures developed toward particulate vaccines. It focuses on the impact of microbe mimicry (e.g. size, charge, hydrophobicity, and surface chemistry) on modulation of the nanoparticles' delivery, trafficking, and targeting antigen-presenting cells to elicit potent humoral and cellular immune responses. This review also provides up-to-date progresses on rational designs of a wide variety of polymeric nanostructures that are loaded with antigens and immunostimulatory molecules, ranging from particles, micelles, nanogels, and polymersomes to advanced core-shell structures where polymeric particles are coated with lipids, cell membranes, or proteins.
Keywords: Cellular immunity; Humoral immunity; Nanoparticle vaccine; Polymer nanostructure; Self-assembly; Subunit vaccine.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures





References
-
- Patz J.A., Campbell-Lendrum D., Holloway T., Foley J.A. Impact of regional climate change on human health. Nature. 2005;438(7066):310–317. - PubMed
-
- Boni M.F., Lemey P., Jiang X., Lam T.T.-Y., Perry B.W., Castoe T.A., Rambaut A., Robertson D.L. Evolutionary origins of the SARS-CoV-2 sarbecovirus lineage responsible for the COVID-19 pandemic. Nat. Microbiol. 2020;5(11):1408–1417. - PubMed
-
- Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y., Ren R., Leung K.S.M., Lau E.H.Y., Wong J.Y., Xing X., Xiang N., Wu Y., Li C., Chen Q., Li D., Liu T., Zhao J., Liu M., Tu W., Chen C., Jin L., Yang R., Wang Q., Zhou S., Wang R., Liu H., Luo Y., Liu Y., Shao G., Li H., Tao Z., Yang Y., Deng Z., Liu B., Ma Z., Zhang Y., Shi G., Lam T.T.Y., Wu J.T., Gao G.F., Cowling B.J., Yang B., Leung G.M., Feng Z. Early transmission dynamics in Wuhan, China, of novel Coronavirus–infected pneumonia. N. Engl. J. Med. 2020;382(13):1199–1207. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

