Monitoring Plasmodium vivax resistance to antimalarials: Persisting challenges and future directions
- PMID: 33360105
- PMCID: PMC7770540
- DOI: 10.1016/j.ijpddr.2020.12.001
Monitoring Plasmodium vivax resistance to antimalarials: Persisting challenges and future directions
Abstract
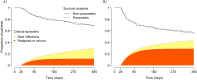
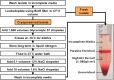
Emerging antimalarial drug resistance may undermine current efforts to control and eliminate Plasmodium vivax, the most geographically widespread yet neglected human malaria parasite. Endemic countries are expected to assess regularly the therapeutic efficacy of antimalarial drugs in use in order to adjust their malaria treatment policies, but proper funding and trained human resources are often lacking to execute relatively complex and expensive clinical studies, ideally complemented by ex vivo assays of drug resistance. Here we review the challenges for assessing in vivo P. vivax responses to commonly used antimalarials, especially chloroquine and primaquine, in the presence of confounding factors such as variable drug absorption, metabolism and interaction, and the risk of new infections following successful radical cure. We introduce a simple modeling approach to quantify the relative contribution of relapses and new infections to recurring parasitemias in clinical studies of hypnozoitocides. Finally, we examine recent methodological advances that may render ex vivo assays more practical and widely used to confirm P. vivax drug resistance phenotypes in endemic settings and review current approaches to the development of robust genetic markers for monitoring chloroquine resistance in P. vivax populations.
Keywords: Chloroquine; Clinical studies; Drug resistance; Ex vivo assays; Molecular markers; Plasmodium vivax; Primaquine.
Copyright © 2020 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
The authors of the manuscript “Monitoring
Figures

References
-
- Abreha T., Hwang J., Thriemer K., Tadesse Y., Girma S., Melaku Z., Assef A., Kassa M., Chatfield M.D., Landman K.Z., Chenet S.M., Lucchi N.W., Udhayakumar V., Zhou Z., Shi Y.P., Kachur S.P., Jima D., Kebede A., Solomon H., Mekasha A., Alemayehu B.H., Malone J.L., Dissanayake G., Teka H., Auburn S., von Seidlein L., Price R.N. Comparison of artemether-lumefantrine and chloroquine with and without primaquine for the treatment of Plasmodium vivax infection in Ethiopia: a randomized controlled trial. PLoS Med. 2017;14 - PMC - PubMed
-
- Almeida A.C., Elias A.B.R., Marques M.P., de Melo G.C., da Costa A.G., Figueiredo E.F.G., Brasil L.W., Rodrigues-Soares F., Monteiro W.M., de Lacerda M.V.G., Lanchote V.L., Suarez-Kurtz G. Impact of Plasmodium vivax malaria and antimalarial treatment on cytochrome P450 activity in Brazilian patients. Br. J. Clin. Pharmacol. 2020 doi: 10.1111/bcp.14574. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

