Host transcriptome response to Borrelia burgdorferi sensu lato
- PMID: 33360384
- PMCID: PMC8127729
- DOI: 10.1016/j.ttbdis.2020.101638
Host transcriptome response to Borrelia burgdorferi sensu lato
Abstract
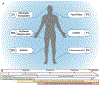
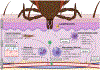
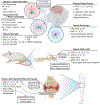
The host immune response to infection is a well-coordinated system of innate and adaptive immune cells working in concert to prevent the colonization and dissemination of a pathogen. While this typically leads to a beneficial outcome and the suppression of disease pathogenesis, the Lyme borreliosis bacterium, Borrelia burgdorferi sensu lato, can elicit an immune profile that leads to a deleterious state. As B. burgdorferi s.l. produces no known toxins, it is suggested that the immune and inflammatory response of the host are responsible for the manifestation of symptoms, including flu-like symptoms, musculoskeletal pain, and cognitive disorders. The past several years has seen a substantial increase in the use of microarray and sequencing technologies to investigate the transcriptome response induced by B. burgdorferi s.l., thus enabling researchers to identify key factors and pathways underlying the pathophysiology of Lyme borreliosis. In this review we present the major host transcriptional outcomes induced by the bacterium across several studies and discuss the overarching theme of the host inflammatory and immune response, and how it influences the pathology of Lyme borreliosis.
Keywords: Borrelia burgdorferi; Borreliosis; Host transcriptome; Lyme.
Copyright © 2020 The Author(s). Published by Elsevier GmbH.. All rights reserved.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

