Long-term safety of pembrolizumab monotherapy and relationship with clinical outcome: A landmark analysis in patients with advanced melanoma
- PMID: 33360855
- PMCID: PMC8388128
- DOI: 10.1016/j.ejca.2020.11.010
Long-term safety of pembrolizumab monotherapy and relationship with clinical outcome: A landmark analysis in patients with advanced melanoma
Abstract
Objective: Long-term safety of pembrolizumab in melanoma was analyzed in KEYNOTE-001, KEYNOTE-002, and KEYNOTE-006.
Patients and methods: Analysis involved patients who received ≥1 pembrolizumab dose. Lead-time bias was addressed via landmark analyses in patients who were progression-free before day 147.
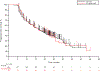
Results: Adverse events (AEs) were analyzed for 1567 patients (median follow-up, 42.4 months). Most AEs were mild/moderate; grade 3/4 treatment-related AEs occurred in 17.7% of patients. Two pembrolizumab-related deaths occurred. Any-grade immune-mediated AEs (imAEs) occurred in 23.0%, most commonly hypothyroidism (9.1%), pneumonitis (3.3%), and hyperthyroidism (3.0%); grade 3/4 imAEs occurred in 6.9% of patients. Most imAEs occurred within 16 weeks of treatment. In landmark analysis, patients who did (n = 79) versus did not (n = 384) develop imAEs had similar objective response rates (ORRs) (64.6% versus 63.0%); median time to response (TTR), 5.6 months for both; median duration of response (DOR), 20.0 versus 25.3 months; median progression-free survival (PFS), 17.0 versus 17.7 months; median overall survival (OS), not reached (NR) versus 43 months (p = 0.1104). Patients who did (n = 17) versus did not (n = 62) receive systemic corticosteroids had similar ORRs (70.6% vs. 62.9%) and median TTR (6.4 vs. 5.6 months) but numerically shorter median PFS (9.9 vs. 17.0 months); median DOR, 14.2 months versus NR; median OS, NR for both.
Conclusions: These results enhance the knowledge base for pembrolizumab in advanced melanoma, with no new toxicity signals after lengthy follow-up of a large population. In landmark analyses, pembrolizumab efficacy was similar regardless of imAEs or systemic corticosteroid use.
Clinical trial registry: NCT01295827, NCT01704287, NCT01866319.
Keywords: Advanced melanoma; Corticosteroid use; Immune-checkpoint inhibitors; Immune-related adverse events; Immunomodulating drugs; PD-1 inhibitors; Pembrolizumab.
Copyright © 2020 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Conflict of interest statement C.R. has participated in advisory boards for Roche; Pierre Fabre; Merck; Novartis; Amgen; Bristol-Myers Squibb; Novartis; Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, U.S.A. (MSD); and Sanofi. O.H. reports personal fees from Merck for consulting during the conduct of the study; as well as contracted research for his institution from Arcus, Aduro, Akeso, Amgen, Array, Bristol-Myers Squibb, CytomX, Exelixis, Genentech, GSK, Immunocore, Incyte, Iovance, Merck, Moderna, Merck Serono, NextCure, Novartis, Regeneron, Roche, Seattle Genetics, Torque, and Zelluna outside the submitted work. A.R. has received personal fees as honoraria for consulting from Amgen, Chugai, Genentech-Roche, Novartis, and Merck; and personal fees, as a scientific advisory member and stockholder from Arcus, Bi-oncotech, Compugen, CytomX, Five Prime, FLX-Bio, Merus, Rgenix, PACT Pharma, and Tango Therapeutics, outside the submitted work. J.S.W. reports personal fees for honoraria for advisory boards and transportation from Merck, Bristol-Myers Squibb, Genentech, Celldex, Pfizer, and AstraZeneca, outside the submitted work. In addition. J.S.W. has a patent named on a PD-1 biomarker patent by Biodesix issued. I.D. reports grants from Merck, Bristol-Myers Squibb, Incyte, OncoSec, and Regeneron, and personal fees from Regeneron, during the conduct of the study. F.S.H. reports other from Merck to their institution for clinical trial support during the conduct of the study; grants, personal fees, and consulting from Bristol-Myers Squibb; personal fees from Merck, EMD Serono, Genentech/Roche, Bayer, Partners Therapeutics, Sanofi, Pfizer, and Kairos for consulting; grants and personal fees from Novartis for consulting; personal fees from Takeda, Surface, Compass Therapeutics, Verastem, and Rheos for advisory boards; personal fees from Apricity and Bicara for scientific advisory board and equity; personal fees from Aduro for advisor consulting; personal fees from Pionyr for advisory board and equity; personal fees from 7 Hills Pharma for advisor; other from Torque for scientific advisory board and equity; outside the submitted work. In addition, F.S.H. has a patent for Methods for Treating MICA-Related Disorders (#20100111973) with royalties paid, a patent for Tumor antigens and uses thereof issued (#7250291), a patent for Angiopoieten-2 Biomarkers Predictive of Anti-immune checkpoint response pending (#20170248603), a patent for Compositions and Methods for Identification, Assessment, Prevention, and Treatment of Melanoma using PD-L1 Isoforms pending (#20160340407), five patents for Therapeutic peptides pending (#20160046716, #20140004112, #20170022275, #20170008962, #9402905), and a patent for “methods of using pembrolizumab and trebananib.” J.D.W. reports personal fees for consulting from Adaptive Biotech, Amgen, Apricity, Ascentage Pharma, Astellas, AstraZeneca, Bayer, BeiGene, Bristol-Myers Squibb, Celgene, Chugai, Eli Lilly, F-star, Imvaq, Kyowa Hakko Kirin, Linnaeus, MedImmune, Merck, Neon Therapeutics, Ono, Polaris Pharma, Polynoma, PsiOxus, Puretech, Recepta, Takara Bio, Trieza, Truvax, Serametrix, Surface Oncology, Syndax, and Syntalogic; research support from Bristol-Myers Squibb and AstraZeneca; and equity in Potenza Therapeutics, Tizona Pharmaceuticals, Adaptive Biotechnologies, Imvaq, BeiGene, Trieza, and Linnaeus. T.C.M. reports personal fees for honorarium from Bristol-Myers Squibb, Aduro, Merck, and Incyte outside the submitted work. R.W.J. reports other from Bristol-Myers Squibb, and Gilead for consulting, outside the submitted work. C.B. reports personal fees from Bristol-Myers Squibb (board advisor), speaker fees from Merck, and travel fees from Amgen and Sandoz outside the submitted work. GVL is consultant advisor for Aduro Biotech Inc, Amgen Inc, Array Biopharma inc, Boehringer Ingelheim International GmbH, Bristol-Myers Squibb, Highlight Therapeutics S.L., MSD, Novartis Pharma AG, Pierre Fabre, QBiotics Group Limited, Regeneron Pharmaceuticals Inc, SkylineDX B.V. I.P. is a consultant for Amgen, Merck, and Iovance, outside the submitted work. R.Dummer reports intermittent, project focused consulting and/or advisory relationships with Novartis, MSD, Bristol-Myers Squibb, Roche, Amgen, Takeda, Pierre Fabre, Sun Pharma, Sanofi, Catalym, Second Genome, Regeneron, Alligator, MaxiVAX SA and touchIME outside the submitted work. J.L. reports employment at MSD, and GSK. S.J.D. reports personal fees as an employee of MSD, and shareholder inMerck & Co., Inc., Kenilworth, NJ, USA. M.S.C. has served on advisory boards for Bristol-Myers Squibb, MSD, Amgen, Novartis, Pierre Fabre, Roche, Sanofi, Merck, Ideaya, Regeneron, Nektar, Eisai, Oncosec and Qbiotics., outside the submitted work. A.J. reports consulting and/or advisory role for Neolukin, Janssen Oncology, Ipsen, AstraZeneca, Sanofi, Noxopharm, IQvia, Pfizer, Novartis, Bristol-Myers Squibb, and Merck Serono, outside the submitted work. A.J. also reports research funding from Bristol-myers Squibb, Janssen Oncology, MSD, Mayne Pharma, Roche/Genentech, Bayer, Macrogenics, Lilly, Pfizer, AstraZeneca, and Corvus Pharmaceuticals. All remaining authors have declared no conflicts of interest.
Figures

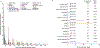
References
-
- KEYTRUDA® (pembrolizumab) for injection, for intravenous use. 11/2020. Merck Sharp & Dohme Corp. Whitehouse Station, NJ, USA; 2020.
-
- Hamid O, Puzanov I, Dummer R, Schachter A, Daud A, Schadendorf D, et al. Final analysis of a randomized trial comparing pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory advanced melanoma. Eur J Canc 2017;86:37–45. - PubMed
-
- Robert C, Ribas A, Schachter J, Arance A, Grob JJ, Mortier L, et al. Pembrolizumab versus ipilimumab in advanced melanoma (KEYNOTE-006): post-hoc 5-year results from an open-label, multicentre, randomised, controlled, phase 3 study. Lancet Oncol 2019;20(9):1239–51. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

