Biochemical and crystallographic investigations into isonitrile formation by a nonheme iron-dependent oxidase/decarboxylase
- PMID: 33361191
- PMCID: PMC7949033
- DOI: 10.1074/jbc.RA120.015932
Biochemical and crystallographic investigations into isonitrile formation by a nonheme iron-dependent oxidase/decarboxylase
Abstract
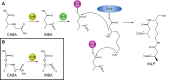
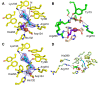
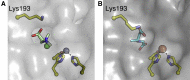
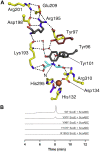
The isonitrile moiety is found in marine sponges and some microbes, where it plays a role in processes such as virulence and metal acquisition. Until recently only one route was known for isonitrile biosynthesis, a condensation reaction that brings together a nitrogen atom of l-Trp/l-Tyr with a carbon atom from ribulose-5-phosphate. With the discovery of ScoE, a mononuclear Fe(II) α-ketoglutarate-dependent dioxygenase from Streptomyces coeruleorubidus, a second route was identified. ScoE forms isonitrile from a glycine adduct, with both the nitrogen and carbon atoms coming from the same glycyl moiety. This reaction is part of the nonribosomal biosynthetic pathway of isonitrile lipopeptides. Here, we present structural, biochemical, and computational investigations of the mechanism of isonitrile formation by ScoE, an unprecedented reaction in the mononuclear Fe(II) α-ketoglutarate-dependent dioxygenase superfamily. The stoichiometry of this enzymatic reaction is measured, and multiple high-resolution (1.45-1.96 Å resolution) crystal structures of Fe(II)-bound ScoE are presented, providing insight into the binding of substrate, (R)-3-((carboxylmethyl)amino)butanoic acid (CABA), cosubstrate α-ketoglutarate, and an Fe(IV)=O mimic oxovanadium. Comparison to a previously published crystal structure of ScoE suggests that ScoE has an "inducible" α-ketoglutarate binding site, in which two residues arginine-157 and histidine-299 move by approximately 10 Å from the surface of the protein into the active site to create a transient α-ketoglutarate binding pocket. Together, data from structural analyses, site-directed mutagenesis, and computation provide insight into the mode of α-ketoglutarate binding, the mechanism of isonitrile formation, and how the structure of ScoE has been adapted to perform this unusual chemical reaction.
Keywords: crystallography; enzyme mechanism; molecular dynamics; mononuclear Fe(II) α-ketoglutarate dependent dioxygenase; stoichiometry; structure function.
Copyright © 2021 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflicts of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures



Similar articles
-
Probing the Mechanism of Isonitrile Formation by a Non-Heme Iron(II)-Dependent Oxidase/Decarboxylase.J Am Chem Soc. 2022 Apr 6;144(13):5893-5901. doi: 10.1021/jacs.1c12891. Epub 2022 Mar 7. J Am Chem Soc. 2022. PMID: 35254829 Free PMC article.
-
Biosynthesis of isonitrile lipopeptides.Curr Opin Chem Biol. 2024 Aug;81:102470. doi: 10.1016/j.cbpa.2024.102470. Epub 2024 May 23. Curr Opin Chem Biol. 2024. PMID: 38788523 Free PMC article. Review.
-
Crystal structure of the non-heme iron dioxygenase PtlH in pentalenolactone biosynthesis.J Biol Chem. 2007 Dec 14;282(50):36552-60. doi: 10.1074/jbc.M706358200. Epub 2007 Oct 16. J Biol Chem. 2007. PMID: 17942405 Free PMC article.
-
The interplay of protein-ligand and water-mediated interactions shape affinity and selectivity in the LAO binding protein.FEBS J. 2020 Feb;287(4):763-782. doi: 10.1111/febs.15019. Epub 2019 Aug 5. FEBS J. 2020. PMID: 31348608
-
The common chemical logic of 'bridged' peroxo species in mononuclear non-heme iron systems.Crit Rev Biochem Mol Biol. 2024 Dec;59(6):418-433. doi: 10.1080/10409238.2025.2455084. Epub 2025 Jan 29. Crit Rev Biochem Mol Biol. 2024. PMID: 39878573 Review.
Cited by
-
Optimized Substrate Positioning Enables Switches in the C-H Cleavage Site and Reaction Outcome in the Hydroxylation-Epoxidation Sequence Catalyzed by Hyoscyamine 6β-Hydroxylase.J Am Chem Soc. 2024 Sep 4;146(35):24271-24287. doi: 10.1021/jacs.4c04406. Epub 2024 Aug 22. J Am Chem Soc. 2024. PMID: 39172701 Free PMC article.
-
Isonitrile biosynthesis by non-heme iron(II)-dependent oxidases/decarboxylases.Methods Enzymol. 2024;704:143-172. doi: 10.1016/bs.mie.2024.06.002. Epub 2024 Jun 29. Methods Enzymol. 2024. PMID: 39300646 Free PMC article.
-
Probing the Mechanism of Isonitrile Formation by a Non-Heme Iron(II)-Dependent Oxidase/Decarboxylase.J Am Chem Soc. 2022 Apr 6;144(13):5893-5901. doi: 10.1021/jacs.1c12891. Epub 2022 Mar 7. J Am Chem Soc. 2022. PMID: 35254829 Free PMC article.
-
Biosynthesis of macrolactam antibiotics with β-amino acid polyketide starter units.J Antibiot (Tokyo). 2024 Aug;77(8):486-498. doi: 10.1038/s41429-024-00742-2. Epub 2024 May 30. J Antibiot (Tokyo). 2024. PMID: 38816450 Free PMC article. Review.
-
Biosynthesis of isonitrile lipopeptides.Curr Opin Chem Biol. 2024 Aug;81:102470. doi: 10.1016/j.cbpa.2024.102470. Epub 2024 May 23. Curr Opin Chem Biol. 2024. PMID: 38788523 Free PMC article. Review.
References
-
- Brady S.F., Clardy J. Cloning and heterologous expression of isocyanide biosynthetic genes from environmental DNA. Angew. Chem. Int. Ed. Engl. 2005;44:7063–7065. - PubMed
-
- Harris N.C., Sato M., Herman N.A., Twigg F., Cai W., Liu J., Zhu X., Downey J., Khalaf R., Martin J., Koshino H., Zhang W. Biosynthesis of isonitrile lipopeptides by conserved nonribosomal peptide synthetase gene clusters in actinobacteria. Proc. Natl. Acad. Sci. U. S. A. 2017;114:7025–7030. - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

