A Critical Role for Na+/H+ Exchanger Regulatory Factor 1 in Modulating FcεRI-Mediated Mast Cell Activation
- PMID: 33361207
- PMCID: PMC7855521
- DOI: 10.4049/jimmunol.2000671
A Critical Role for Na+/H+ Exchanger Regulatory Factor 1 in Modulating FcεRI-Mediated Mast Cell Activation
Abstract
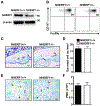
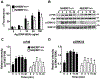
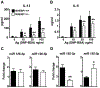
Mast cells are tissue-resident immune cells that play pivotal roles in initiating and amplifying allergic/anaphylactic reactions in humans. Their activation occurs via multiple mechanisms, which include cross-linking of the IgE-bound, high-affinity IgE receptors (FcεRI) by allergens or Ags and the binding of anaphylatoxins such as C3a to its receptor, C3aR. We have previously demonstrated that the Na+/H+ exchanger regulatory factor 1 (NHERF1) promotes C3aR functions in human mast cells. In the current study, we show that NHERF1 regulates mast cell response following FcεRI stimulation. Specifically, intracellular Ca2+ mobilization, activation of the MAPKs (ERK1/2 and P38), and production of cytokines (IL-13 and IL-6) following exposure to IgE/Ag were significantly reduced in mast cells from NHERF1+/‒ mice. In agreement with our in vitro data, mast cell-mediated passive cutaneous anaphylaxis and passive systemic anaphylaxis were reduced in NHERF1+/‒ mice and mast cell-deficient KitW-sh/W-sh mice engrafted with NHERF1+/‒ mast cells. Mechanistically, the levels of microRNAs (miRNAs) that regulate mast cell responses, miRNA 155-3p and miRNA 155-5p, were altered in mast cells from NHERF1+/‒ mice. Moreover, NHERF1 rapidly localized to the nucleus of mast cells following FcεRI stimulation. In summary, our results suggest that the NHERF1 acts as an adapter molecule and promotes IgE/Ag-induced mast cell activation. Further elucidating the mechanisms through which NHERF1 modulates mast cell responses will lend insights into the development of new therapeutic strategies to target mast cells during anaphylaxis or other allergic diseases.
Copyright © 2021 by The American Association of Immunologists, Inc.
Conflict of interest statement
CONFLICT OF INTEREST
The authors have no conflict of interest to declare.
Figures
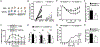


References
-
- DeConde AS, and Soler ZM. 2016. Chronic rhinosinusitis: Epidemiology and burden of disease. Am J Rhinol Allergy 30: 134–139. - PubMed
-
- Papi A, Brightling C, Pedersen SE, and Reddel HK. 2018. Asthma. Lancet 391: 783–800. - PubMed
-
- Saito H, Ishizaka T, and Ishizaka K. 2013. Mast cells and IgE: from history to today. Allergol Int 62: 3–12. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

