Low Vitamin D Status Is Associated with Inflammation in Patients with Chronic Obstructive Pulmonary Disease
- PMID: 33361208
- PMCID: PMC7812059
- DOI: 10.4049/jimmunol.2000964
Low Vitamin D Status Is Associated with Inflammation in Patients with Chronic Obstructive Pulmonary Disease
Abstract
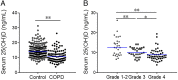
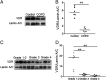
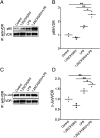
Vitamin D deficiency is associated with increased risks of chronic obstructive pulmonary disease (COPD). Nevertheless, the mechanisms remain unknown. This study analyzed the correlations between vitamin D levels and inflammation in COPD patients. One hundred and one patients with COPD and 202 control subjects were enrolled. Serum 25(OH)D level and inflammatory cytokines were detected. Serum 25(OH)D was decreased and inflammatory cytokines were increased in COPD patients. According to forced expiratory volume in 1 s, COPD patients were divided into three grades. Furthermore, serum 25(OH)D was gradually decreased in COPD patients ranging from grade 1-2 to 4. Serum 25(OH)D was inversely associated with inflammatory cytokines in COPD patients. Further analysis found that NF-κB and AP-1 signaling were activated in COPD patients. Besides, inflammatory signaling was gradually increased in parallel with the severity of COPD. By contrast, pulmonary nuclear vitamin D receptor was decreased in COPD patients. In vitro experiments showed that 1,25(OH)2D3 inhibited LPS-activated inflammatory signaling in A549 cells (human lung adenocarcinoma cell). Mechanically, 1,25(OH)2D3 reinforced physical interactions between vitamin D receptor with NF-κB p65 and c-Jun. Our results indicate that vitamin D is inversely correlated with inflammatory signaling in COPD patients. Inflammation may be a vital mediator of COPD progress in patients with low vitamin D levels.
Copyright © 2021 by The American Association of Immunologists, Inc.
Conflict of interest statement
The authors have no financial conflicts of interest.
Figures





Similar articles
-
Associations of Vitamin D With GPX4 and Iron Parameters in Chronic Obstructive Pulmonary Disease Patients: A Case-Control Study.Can Respir J. 2024 Oct 28;2024:4505905. doi: 10.1155/2024/4505905. eCollection 2024. Can Respir J. 2024. PMID: 39502871 Free PMC article.
-
Low Vitamin D Status Is Associated with Epithelial-Mesenchymal Transition in Patients with Chronic Obstructive Pulmonary Disease.J Immunol. 2019 Sep 15;203(6):1428-1435. doi: 10.4049/jimmunol.1900229. Epub 2019 Aug 19. J Immunol. 2019. PMID: 31427443
-
Experimental study on 1,25(OH)2 D3 amelioration of oral lichen planus through regulating NF-κB signaling pathway.Oral Dis. 2017 Sep;23(6):770-778. doi: 10.1111/odi.12659. Epub 2017 Mar 30. Oral Dis. 2017. PMID: 28231625
-
The role of vitamin D in pulmonary disease: COPD, asthma, infection, and cancer.Respir Res. 2011 Mar 18;12(1):31. doi: 10.1186/1465-9921-12-31. Respir Res. 2011. PMID: 21418564 Free PMC article. Review.
-
Vitamin D modulates airway smooth muscle function in COPD.Curr Opin Pharmacol. 2012 Jun;12(3):266-74. doi: 10.1016/j.coph.2012.01.014. Epub 2012 Feb 25. Curr Opin Pharmacol. 2012. PMID: 22365730 Review.
Cited by
-
rs7041 and rs4588 Polymorphisms in Vitamin D Binding Protein Gene (VDBP) and the Risk of Diseases.Int J Mol Sci. 2022 Jan 15;23(2):933. doi: 10.3390/ijms23020933. Int J Mol Sci. 2022. PMID: 35055118 Free PMC article. Review.
-
Using system biology and bioinformatics to identify the influences of COVID-19 co-infection with influenza virus on COPD.Funct Integr Genomics. 2023 May 24;23(2):175. doi: 10.1007/s10142-023-01091-3. Funct Integr Genomics. 2023. PMID: 37221323 Free PMC article.
-
Associations of Vitamin D With GPX4 and Iron Parameters in Chronic Obstructive Pulmonary Disease Patients: A Case-Control Study.Can Respir J. 2024 Oct 28;2024:4505905. doi: 10.1155/2024/4505905. eCollection 2024. Can Respir J. 2024. PMID: 39502871 Free PMC article.
-
Protective factors against oxidative stress in COPD: focus on Nrf2-dependent antioxidant gene expression.Front Med (Lausanne). 2025 May 2;12:1492256. doi: 10.3389/fmed.2025.1492256. eCollection 2025. Front Med (Lausanne). 2025. PMID: 40385585 Free PMC article. Review.
-
Correlations among Pulmonary DJ-1, VDR and Nrf-2 in patients with Chronic Obstructive Pulmonary Disease: A Case-control Study.Int J Med Sci. 2021 Apr 22;18(11):2449-2456. doi: 10.7150/ijms.58452. eCollection 2021. Int J Med Sci. 2021. PMID: 33967623 Free PMC article.
References
-
- Vestbo J., Hurd S. S., Agustí A. G., Jones P. W., Vogelmeier C., Anzueto A., Barnes P. J., Fabbri L. M., Martinez F. J., Nishimura M., et al. 2013. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am. J. Respir. Crit. Care Med. 187: 347–365. - PubMed
-
- Celli B. R., Halbert R. J., Nordyke R. J., Schau B. 2005. Airway obstruction in never smokers: results from the third national health and nutrition examination survey. Am. J. Med. 118: 1364–1372. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

