Piperaquine Pharmacokinetics during Intermittent Preventive Treatment for Malaria in Pregnancy
- PMID: 33361303
- PMCID: PMC8092554
- DOI: 10.1128/AAC.01150-20
Piperaquine Pharmacokinetics during Intermittent Preventive Treatment for Malaria in Pregnancy
Abstract
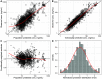
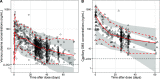
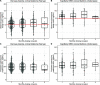
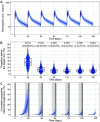
Dihydroartemisinin-piperaquine (DP) is a long-acting artemisinin combination treatment that provides effective chemoprevention and has been proposed as an alternative antimalarial drug for intermittent preventive therapy in pregnancy (IPTp). Several pharmacokinetic studies have shown that dose adjustment may not be needed for the treatment of malaria in pregnancy with DP. However, there are limited data on the optimal dosing for IPTp. This study aimed to evaluate the population pharmacokinetics of piperaquine given as IPTp in pregnant women. Pregnant women were enrolled in clinical trials conducted in Kenya and Indonesia and treated with standard 3-day courses of DP, administered in 4- to 8-week intervals from the second trimester until delivery. Pharmacokinetic blood samples were collected for piperaquine drug measurements before each treatment round, at the time of breakthrough symptomatic malaria, and at delivery. Piperaquine population pharmacokinetic properties were investigated using nonlinear mixed-effects modeling with a prior approach. In total, data from 366 Kenyan and 101 Indonesian women were analyzed. The pharmacokinetic properties of piperaquine were adequately described using a flexible transit absorption (n = 5) followed by a three-compartment disposition model. Gestational age did not affect the pharmacokinetic parameters of piperaquine. After three rounds of monthly IPTp, 9.45% (95% confidence interval [CI], 1.8 to 26.5%) of pregnant women had trough piperaquine concentrations below the suggested target concentration (10.3 ng/ml). Translational simulations suggest that providing the full treatment course of DP at monthly intervals provides sufficient protection to prevent malaria infection. Monthly administration of DP has the potential to offer optimal prevention of malaria during pregnancy. (This study has been registered at ClinicalTrials.gov under identifier NCT01669941 and in the ISRCTN under number ISRCTN34010937.).
Keywords: dihydroartemisinin-piperaquine; intermittent preventive treatment in pregnancy; nonlinear mixed-effects modeling; population pharmacokinetic model.
Copyright © 2021 Chotsiri et al.
Figures
References
-
- Lwin KM, Phyo AP, Tarning J, Hanpithakpong W, Ashley EA, Lee SJ, Cheah P, Singhasivanon P, White NJ, Lindegardh N, Nosten F. 2012. Randomized, double-blind, placebo-controlled trial of monthly versus bimonthly dihydroartemisinin-piperaquine chemoprevention in adults at high risk of malaria. Antimicrob Agents Chemother 56:1571–1577. doi:10.1128/AAC.05877-11. - DOI - PMC - PubMed
-
- Nankabirwa JI, Wandera B, Amuge P, Kiwanuka N, Dorsey G, Rosenthal PJ, Brooker SJ, Staedke SG, Kamya MR. 2014. Impact of intermittent preventive treatment with dihydroartemisinin-piperaquine on malaria in Ugandan schoolchildren: a randomized, placebo-controlled trial. Clin Infect Dis 58:1404–1412. doi:10.1093/cid/ciu150. - DOI - PMC - PubMed
-
- Chotsiri P, Zongo I, Milligan P, Compaore YD, Somé AF, Chandramohan D, Hanpithakpong W, Nosten F, Greenwood B, Rosenthal PJ, White NJ, Ouédraogo J-B, Tarning J. 2019. Optimal dosing of dihydroartemisinin-piperaquine for seasonal malaria chemoprevention in young children. Nat Commun 10:480. doi:10.1038/s41467-019-08297-9. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

