Predicting the Disposition of the Antimalarial Drug Artesunate and Its Active Metabolite Dihydroartemisinin Using Physiologically Based Pharmacokinetic Modeling
- PMID: 33361307
- PMCID: PMC8092529
- DOI: 10.1128/AAC.02280-20
Predicting the Disposition of the Antimalarial Drug Artesunate and Its Active Metabolite Dihydroartemisinin Using Physiologically Based Pharmacokinetic Modeling
Abstract
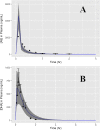
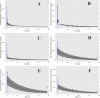
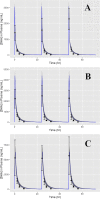
Artemisinin-based combination therapies (ACTs) have proven to be effective in helping to combat the global malaria epidemic. To optimally apply these drugs, information about their tissue-specific disposition is required, and one approach to predict these pharmacokinetic characteristics is physiologically based pharmacokinetic (PBPK) modeling. In this study, a whole-body PBPK model was developed to simulate the time-dependent tissue concentrations of artesunate (AS) and its active metabolite, dihydroartemisinin (DHA). The model was developed for both rats and humans and incorporated drug metabolism of the parent compound and major metabolite. Model calibration was conducted using data from the literature in a Bayesian framework, and model verification was assessed using separate sets of data. Results showed good agreement between model predictions and the validation data, demonstrating the capability of the model in predicting the blood, plasma, and tissue pharmacokinetics of AS and DHA. It is expected that such a tool will be useful in characterizing the disposition of these chemicals and ultimately improve dosing regimens by enabling a quantitative assessment of the tissue-specific drug levels critical in the evaluation of efficacy and toxicity.
Keywords: PBPK; antimalarial agents; artemisinin; malaria; modeling.
Copyright © 2021 American Society for Microbiology.
Figures


References
-
- World Health Organization. 2015. Guidelines for the treatment of malaria. World Health Organization, Geneva, Switzerland.
-
- Morris CA, Duparc S, Borghini-Fuhrer I, Jung D, Shin CS, Fleckenstein L. 2011. Review of the clinical pharmacokinetics of artesunate and its active metabolite dihydroartemisinin following intravenous, intramuscular, oral or rectal administration. Malar J 10:263. doi: 10.1186/1475-2875-10-263. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

