Acinetobacter baumannii Targets Human Carcinoembryonic Antigen-Related Cell Adhesion Molecules (CEACAMs) for Invasion of Pneumocytes
- PMID: 33361319
- PMCID: PMC7762790
- DOI: 10.1128/mSystems.00604-20
Acinetobacter baumannii Targets Human Carcinoembryonic Antigen-Related Cell Adhesion Molecules (CEACAMs) for Invasion of Pneumocytes
Abstract
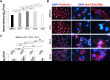
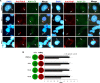
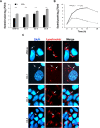
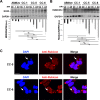
Multidrug-resistant Acinetobacter baumannii is regarded as a life-threatening pathogen mainly associated with nosocomial and community-acquired pneumonia. Here, we show that A. baumannii can bind the human carcinoembryonic antigen-related cell adhesion molecule (CEACAM) receptors CEACAM1, CEACAM5, and CEACAM6. This specific interaction enhances A. baumannii internalization in membrane-bound vacuoles, promptly decorated with Rab5, Rab7, and lipidated microtubule-associated protein light chain 3 (LC3). Dissecting intracellular signaling pathways revealed that infected pneumocytes trigger interleukin-8 (IL-8) secretion via the extracellular signal-regulated kinase (ERK)1/2 and nuclear factor-kappa B (NF-κB) signaling pathways for A. baumannii clearance. However, in CEACAM1-L-expressing cells, IL-8 secretion lasts only 24 h, possibly due to an A. baumannii-dependent effect on the CEACAM1-L intracellular domain. Conversely, the glycosylphosphatidylinositol-anchored CEACAM5 and CEACAM6 activate the c-Jun NH2-terminal kinase (JNK)1/2-Rubicon-NOX2 pathway, suggestive of LC3-associated phagocytosis. Overall, our data show for the first time novel mechanisms of adhesion to and invasion of pneumocytes by A. baumannii via CEACAM-dependent signaling pathways that eventually lead to bacterial killing. These findings suggest that CEACAM upregulation could put patients at increased risk of lower respiratory tract infection by A. baumannii IMPORTANCE This work shows for the first time that Acinetobacter baumannii binds to carcinoembryonic antigen-related cell adhesion molecule 1 (CEACAM1), CEACAM5, and CEACAM6. This binding significantly enhances A. baumannii internalization within alveolar host cell epithelia. Intracellular trafficking involves typical Rab5 and Rab7 vacuolar proteins as well as light chain 3 (LC3) and slowly progresses to bacterial killing by endosome acidification. CEACAM engagement by A. baumannii leads to distinct and specific downstream signaling pathways. The CEACAM1 pathway finely tunes interleukin-8 (IL-8) secretion, whereas CEACAM5 and CEACAM6 mediate LC3-associated phagocytosis. The present study provides new insights into A. baumannii-host interactions and could represent a promising therapeutic strategy to reduce pulmonary infections caused by this pathogen.
Keywords: Acinetobacter baumannii; MAPKs; Rubicon; bacterial adhesion/invasion; carcinoembryonic antigen‐related cell adhesion molecules.
Copyright © 2020 Ambrosi et al.
Figures



References
-
- Ambrosi C, Aleandri M, Giordano A, Scribano D, Marazzato M, Zagaglia C, Conte MP, Palamara AT. 2016. Molecular characterisation of extensively drug-resistant Acinetobacter baumannii: first report of a new sequence type in Italy. J Glob Antimicrob Resist 7:154–156. doi: 10.1016/j.jgar.2016.10.002. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

