Kaposi's Sarcoma-Associated Herpesvirus Processivity Factor, ORF59, Binds to Canonical and Linker Histones, and Its Carboxy Terminus Is Dispensable for Viral DNA Synthesis
- PMID: 33361421
- PMCID: PMC8094953
- DOI: 10.1128/JVI.02169-20
Kaposi's Sarcoma-Associated Herpesvirus Processivity Factor, ORF59, Binds to Canonical and Linker Histones, and Its Carboxy Terminus Is Dispensable for Viral DNA Synthesis
Abstract
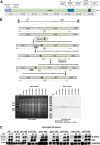
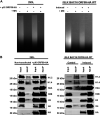
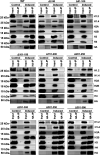
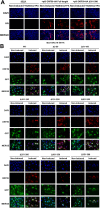
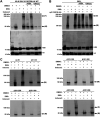
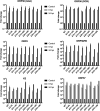
Kaposi's sarcoma-associated herpesvirus (KSHV) is a human oncogenic virus and the causative agent of Kaposi's sarcoma, multicentric Castleman's disease, and primary effusion lymphoma. During lytic reactivation, there is a temporal cascade of viral gene expression that results in the production of new virions. One of the viral factors that is expressed during reactivation is open reading frame 59 (ORF59), the viral DNA polymerase processivity factor. ORF59 plays an essential role for DNA synthesis and is required for the nuclear localization of the viral DNA polymerase (ORF9) to the origin of lytic replication (oriLyt). In addition to its functions in viral DNA synthesis, ORF59 has been shown to interact with chromatin complexes, including histones and cellular methyltransferases. In this study, a series of KSHV BACmids containing 50-amino acid (aa) deletions within ORF59 were generated to determine the interaction domains between ORF59 and histones, as well as to assess the effects on replication fitness as a result of these interactions. These studies show that in the context of infection, ORF59 51 to 100 and 151 to 200 amino acids (aa) are required for interaction with histones, and ORF59 301 to 396 aa are not required for DNA synthesis. Since full-length ORF59 is known to localize to the nucleus, we performed an immunofluorescent assay (IFA) with the ORF59 deletion mutants and showed that all deletions are localized to the nucleus; this includes the ORF59 deletion without the previously identified nuclear localization signal (NLS). These studies further characterize ORF59 and demonstrate its essential role during lytic replication.IMPORTANCE Kaposi's sarcoma-associated herpesvirus (KSHV) is an oncogenic virus and the causative agent of potentially fatal malignancies. Lytic replication of KSHV is an essential part of the viral life cycle, allowing for virus dissemination within the infected host and shedding to infect naive hosts. Viral DNA synthesis is a critical step in the production of new infectious virions. One of the proteins that is vital to this process is open reading frame 59 (ORF59), the viral encoded polymerase processivity factor. Previous work has demonstrated that the function of ORF59 is closely connected to its association with other viral and cellular factors. The studies presented here extend that work to include the interaction between ORF59 and histones. This interaction offers an additional level of regulation of the chromatinized viral genome, ultimately influencing DNA synthesis and transcription dynamics.
Keywords: DNA replication; KSHV; Kaposi's sarcoma-associated herpesvirus; ORF59; histones; processivity factor.
Copyright © 2021 American Society for Microbiology.
Figures

Similar articles
-
The Expression and Nuclear Retention Element of Polyadenylated Nuclear RNA Is Not Required for Productive Lytic Replication of Kaposi's Sarcoma-Associated Herpesvirus.J Virol. 2021 Jun 10;95(13):e0009621. doi: 10.1128/JVI.00096-21. Epub 2021 Jun 10. J Virol. 2021. PMID: 33853955 Free PMC article.
-
The cellular paraspeckle component SFPQ associates with the viral processivity factor ORF59 during lytic replication of Kaposi's Sarcoma-associated herpesvirus (KSHV).Virus Res. 2024 Nov;349:199456. doi: 10.1016/j.virusres.2024.199456. Epub 2024 Sep 7. Virus Res. 2024. PMID: 39214388 Free PMC article.
-
The KSHV ORF20 Protein Interacts with the Viral Processivity Factor ORF59 and Promotes Viral Reactivation.Microbiol Spectr. 2021 Sep 3;9(1):e0014521. doi: 10.1128/Spectrum.00145-21. Epub 2021 Jun 9. Microbiol Spectr. 2021. PMID: 34106579 Free PMC article.
-
Epigenetic regulation of Kaposi's sarcoma-associated herpesvirus replication.Semin Cancer Biol. 2009 Jun;19(3):153-7. doi: 10.1016/j.semcancer.2009.02.010. Epub 2009 Feb 21. Semin Cancer Biol. 2009. PMID: 19429478 Free PMC article. Review.
-
Regulation of KSHV Latency and Lytic Reactivation.Viruses. 2020 Sep 17;12(9):1034. doi: 10.3390/v12091034. Viruses. 2020. PMID: 32957532 Free PMC article. Review.
Cited by
-
The nucleic acid binding protein SFPQ represses EBV lytic reactivation by promoting histone H1 expression.Nat Commun. 2024 May 16;15(1):4156. doi: 10.1038/s41467-024-48333-x. Nat Commun. 2024. PMID: 38755141 Free PMC article.
-
Chinese Giant Salamander Iridovirus 025L Is a Viral Essential Gene.Viruses. 2023 Feb 23;15(3):617. doi: 10.3390/v15030617. Viruses. 2023. PMID: 36992326 Free PMC article.
-
Inhibiting KSHV replication by targeting the essential activities of KSHV processivity protein, PF-8.J Med Virol. 2024 Oct;96(10):e29958. doi: 10.1002/jmv.29958. J Med Virol. 2024. PMID: 39370884 Free PMC article. Review.
-
DNA-Binding Activities of KSHV DNA Polymerase Processivity Factor (PF-8) Complexes.Viruses. 2025 Jan 29;17(2):190. doi: 10.3390/v17020190. Viruses. 2025. PMID: 40006945 Free PMC article.
-
The Expression and Nuclear Retention Element of Polyadenylated Nuclear RNA Is Not Required for Productive Lytic Replication of Kaposi's Sarcoma-Associated Herpesvirus.J Virol. 2021 Jun 10;95(13):e0009621. doi: 10.1128/JVI.00096-21. Epub 2021 Jun 10. J Virol. 2021. PMID: 33853955 Free PMC article.
References
-
- Arvin A, Campadelli-Fiume G, Mocarski E, Moore PS, Roizman B, Whitley R, Yamanishi K. 2007. Human herpesviruses: biology, therapy, and immunoprophylaxis, Cambridge University Press, Cambridge, UK. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

