Diverse Populations of Extracellular Vesicles with Opposite Functions during Herpes Simplex Virus 1 Infection
- PMID: 33361424
- PMCID: PMC8094966
- DOI: 10.1128/JVI.02357-20
Diverse Populations of Extracellular Vesicles with Opposite Functions during Herpes Simplex Virus 1 Infection
Abstract
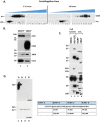
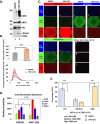
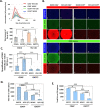
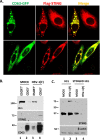
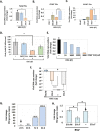
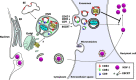
Extracellular vesicles (EVs) are released by all types of cells as a means of intercellular communication. Their significance lies in the fact that they can alter recipient cell functions, despite their limited capacity for cargo. We have previously demonstrated that herpes simplex virus 1 (HSV-1) infection influences the cargo and functions of EVs released by infected cells and that these EVs negatively impact a subsequent HSV-1 infection. In the present study, we have implemented cutting-edge technologies to further characterize EVs released during HSV-1 infection. We identified distinct EV populations that were separable through a gradient approach. One population was positive for the tetraspanin CD63 and was distinct from EVs carrying components of the endosomal sorting complexes required for transport (ESCRT). Nanoparticle tracking analysis (NTA) combined with protein analysis indicated that the production of CD63+ EVs was selectively induced upon HSV-1 infection. The ExoView platform supported these data and suggested that the amount of CD63 per vesicle is larger upon infection. This platform also identified EV populations positive for other tetraspanins, including CD81 and CD9, whose abundance decreased upon HSV-1 infection. The
Keywords: CD63; CD81; ESCRT; HSV-1; L-particles; biogenesis of extracellular vesicles; extracellular vesicles; tetraspanins.
Copyright © 2021 American Society for Microbiology.
Figures

Similar articles
-
HSV-1 virions and related particles: biogenesis and implications in the infection.J Virol. 2025 Mar 18;99(3):e0107624. doi: 10.1128/jvi.01076-24. Epub 2025 Feb 3. J Virol. 2025. PMID: 39898651 Free PMC article. Review.
-
Zika Virus Hijacks Extracellular Vesicle Tetraspanin Pathways for Cell-to-Cell Transmission.mSphere. 2021 Jun 30;6(3):101128msphere0019221. doi: 10.1128/mSphere.00192-21. Epub 2021 Jun 30. mSphere. 2021. PMID: 34190582 Free PMC article.
-
Isolation and characterization of bone mesenchymal cell small extracellular vesicles using a novel mouse model.J Bone Miner Res. 2024 Oct 29;39(11):1633-1643. doi: 10.1093/jbmr/zjae135. J Bone Miner Res. 2024. PMID: 39173022
-
Biogenesis of Extracellular Vesicles during Herpes Simplex Virus 1 Infection: Role of the CD63 Tetraspanin.J Virol. 2019 Jan 4;93(2):e01850-18. doi: 10.1128/JVI.01850-18. Print 2019 Jan 15. J Virol. 2019. PMID: 30355691 Free PMC article.
-
Interventions for prevention of herpes simplex labialis (cold sores on the lips).Cochrane Database Syst Rev. 2015 Aug 7;2015(8):CD010095. doi: 10.1002/14651858.CD010095.pub2. Cochrane Database Syst Rev. 2015. PMID: 26252373 Free PMC article.
Cited by
-
PML Body Component Sp100A Restricts Wild-Type Herpes Simplex Virus 1 Infection.J Virol. 2022 Apr 27;96(8):e0027922. doi: 10.1128/jvi.00279-22. Epub 2022 Mar 30. J Virol. 2022. PMID: 35353002 Free PMC article.
-
Tracing the STING exocytosis pathway during herpes viruses infection.mBio. 2024 Apr 10;15(4):e0037324. doi: 10.1128/mbio.00373-24. Epub 2024 Mar 12. mBio. 2024. PMID: 38470056 Free PMC article.
-
Characterisation of extracellular vesicles in baculovirus infection of Spodoptera frugiperda cells.J Extracell Biol. 2024 Jun 28;3(7):e163. doi: 10.1002/jex2.163. eCollection 2024 Jul. J Extracell Biol. 2024. PMID: 38947876 Free PMC article.
-
Extracellular Vesicles Released by Bovine Alphaherpesvirus 1-Infected A549 Cells May Limit Subsequent Infections of the Progeny Virus.Int J Mol Sci. 2025 Jun 26;26(13):6181. doi: 10.3390/ijms26136181. Int J Mol Sci. 2025. PMID: 40649956 Free PMC article.
-
HSV-1 virions and related particles: biogenesis and implications in the infection.J Virol. 2025 Mar 18;99(3):e0107624. doi: 10.1128/jvi.01076-24. Epub 2025 Feb 3. J Virol. 2025. PMID: 39898651 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

