In-Tree Behavior of Diverse Viruses Harbored in the Chestnut Blight Fungus, Cryphonectria parasitica
- PMID: 33361433
- PMCID: PMC8094943
- DOI: 10.1128/JVI.01962-20
In-Tree Behavior of Diverse Viruses Harbored in the Chestnut Blight Fungus, Cryphonectria parasitica
Abstract
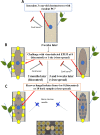
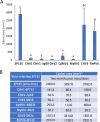
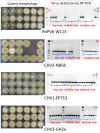
The ascomycete Cryphonectria parasitica causes destructive chestnut blight. Biological control of the fungus by virus infection (hypovirulence) has been shown to be an effective control strategy against chestnut blight in Europe. To provide biocontrol effects, viruses must be able to induce hypovirulence and spread efficiently in chestnut trees. Field studies using living trees to date have focused on a selected family of viruses called hypoviruses, especially prototypic hypovirus CHV1, but there are now known to be many other viruses that infect C. parasitica Here, we tested seven different viruses for their hypovirulence induction, biocontrol potential, and transmission properties between two vegetatively compatible but molecularly distinguishable fungal strains in trees. The test included cytosolically and mitochondrially replicating viruses with positive-sense single-stranded RNA or double-stranded RNA genomes. The seven viruses showed different in planta behaviors and were classified into four groups. Group I, including CHV1, had great biocontrol potential and could protect trees by efficiently spreading and converting virulent to hypovirulent cankers in the trees. Group II could induce high levels of hypovirulence but showed much smaller biocontrol potential, likely because of inefficient virus transmission. Group III showed poor performance in hypovirulence induction and biocontrol, while efficiently being transmitted in the infected trees. Group IV could induce hypovirulence and spread efficiently but showed poor biocontrol potential. Nuclear and mitochondrial genotyping of fungal isolates obtained from the treated cankers confirmed virus transmission between the two fungal strains in most isolates. These results are discussed in view of dynamic interactions in the tripartite pathosystem.IMPORTANCE The ascomycete Cryphonectria parasitica causes destructive chestnut blight, which is controllable by hypovirulence-conferring viruses infecting the fungus. The tripartite chestnut/C. parasitica/virus pathosystem involves the dynamic interactions of their genetic elements, i.e., virus transmission and lateral transfer of nuclear and mitochondrial genomes between fungal strains via anastomosis occurring in trees. Here, we tested diverse RNA viruses for their hypovirulence induction, biocontrol potential, and transmission properties between two vegetatively compatible but molecularly distinguishable fungal strains in live chestnut trees. The tested viruses, which are different in genome type (single-stranded or double-stranded RNA) and organization, replication site (cytosol or mitochondria), virus form (encapsidated or capsidless) and/or symptomatology, have been unexplored in the aforementioned aspects under controlled conditions. This study showed intriguing different in-tree behaviors of the seven viruses and suggested that to exert significant biocontrol effects, viruses must be able to induce hypovirulence and spread efficiently in the fungus infecting the chestnut trees.
Keywords: Cryphonectria parasitica; chestnut blight fungus; hypovirus; mitovirus; mycovirus; reovirus; virus spread.
Copyright © 2021 American Society for Microbiology.
Figures




References
-
- Heiniger U, Rigling D. 1994. Biological control of chestnut blight in Europe. Annu Rev Phytopathol 32:581–599. doi: 10.1146/annurev.py.32.090194.003053. - DOI
Publication types
MeSH terms
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources

