Effect of the use of Galectin-9 and blockade of TIM-3 receptor in the latent cellular reservoir of HIV-1
- PMID: 33361434
- PMCID: PMC8092815
- DOI: 10.1128/JVI.02214-20
Effect of the use of Galectin-9 and blockade of TIM-3 receptor in the latent cellular reservoir of HIV-1
Abstract
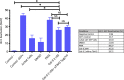
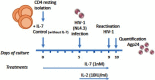
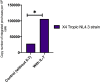
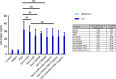
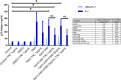
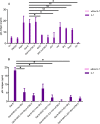
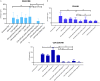
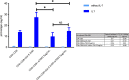
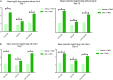
Reactivation of latent HIV-1 is a necessary step for the purging of the viral reservoir, although it does not seem to be enough. The stimulation of HIV-1 specific cytotoxic T lymphocytes (CTL) may be just as essential for this purpose. In this study, we aimed to show the effect of galectin-9 (Gal-9), known to revert HIV-1 latency, in combination with the blockade of TIM-3, a natural receptor for Gal-9 and an exhaustion marker. We confirmed the ability of Gal-9 to reactivate latent HIV-1 in Jurkat-LAT-GFP cells, as well as in an IL-7-based cellular model. This reactivation was not mediated via the TIM-3 receptor, but rather by the recognition of the Gal-9 of a specific oligosaccharide pattern of resting memory CD4+ T cells' surfaces. The potency of Gal-9 in inducing transcription of latent HIV-1 was equal to or greater than that of other latency-reversing agents (LRA). Furthermore, the combination of Gal-9 with other LRA did not show synergistic effects in the reactivation of the latent virus. To evaluate the impact of TIM-3 inhibition on the CTL-response, different co-culture experiments with CD4+T, CD8+ T, and NK cells were performed. Our data showed that blocking TIM-3 was associated with control of viral replication in both in vitro and ex vivo models in cells from PLWH on antiretroviral therapy. A joint strategy of the use of Gal-9 to reactivate latent HIV-1 and the inhibition of TIM-3 to enhance the HIV-1 CTL specific-response was associated with control of the replication of the virus that was being reactivated, thus potentially contributing to the elimination of the viral reservoir. Our results place this strategy as a promising approach to be tested in future studies. Reactivation of latent-HIV-1 by Gal-9 and reinvigoration of CD8+ T cells by TIM-3 blockade could be used separately or in combination.ImportanceHIV-1 infection is a health problem of enormous importance that still causes significant mortality. Antiretroviral treatment (ART) has demonstrated efficacy in the control of HIV-1 replication, decreasing the morbidity and mortality of the infection, but it cannot eradicate the virus. In our work, we tested a protein, galectin-9 (Gal-9), an HIV-1 latency-reversing agent, using an in vitro cellular model of latency and in cells from people living with HIV-1 (PLWH) on antiretroviral therapy. Our results confirmed the potential role of Gal-9 as a molecule with a potent HIV-1 reactivation capacity. More importantly, using a monoclonal antibody against T cell immunoglobulin and the mucin domain-containing molecule 3 (TIM-3) receptor we were able to enhance the HIV-1 cytotoxic T lymphocytes (CTL) specific response to eliminate the CD4+ T cells in which the virus had been reactivated. When used together, i.e., Gal-9 and TIM-3 blockade, control of the replication of HIV-1 was observed, suggesting a decrease in the cellular reservoir.
Copyright © 2020 American Society for Microbiology.
Figures
Similar articles
-
Posttranscriptional Regulation of HIV-1 Gene Expression during Replication and Reactivation from Latency by Nuclear Matrix Protein MATR3.mBio. 2018 Nov 13;9(6):e02158-18. doi: 10.1128/mBio.02158-18. mBio. 2018. PMID: 30425153 Free PMC article.
-
Galectin-9 Mediates HIV Transcription by Inducing TCR-Dependent ERK Signaling.Front Immunol. 2019 Feb 20;10:267. doi: 10.3389/fimmu.2019.00267. eCollection 2019. Front Immunol. 2019. PMID: 30842775 Free PMC article.
-
Influence of galectin-9/Tim-3 interaction on herpes simplex virus-1 latency.J Immunol. 2011 Dec 1;187(11):5745-55. doi: 10.4049/jimmunol.1102105. Epub 2011 Oct 21. J Immunol. 2011. PMID: 22021615 Free PMC article.
-
Transcriptome profiles of latently- and reactivated HIV-1 infected primary CD4+ T cells: A pooled data-analysis.Front Immunol. 2022 Aug 26;13:915805. doi: 10.3389/fimmu.2022.915805. eCollection 2022. Front Immunol. 2022. PMID: 36090997 Free PMC article.
-
The bitter side of sweet: the role of Galectin-9 in immunopathogenesis of viral infections.Rev Med Virol. 2015 May;25(3):175-86. doi: 10.1002/rmv.1832. Epub 2015 Mar 11. Rev Med Virol. 2015. PMID: 25760439 Review.
Cited by
-
Human galectin-9 potently enhances SARS-CoV-2 replication and inflammation in airway epithelial cells.J Mol Cell Biol. 2023 Aug 3;15(4):mjad030. doi: 10.1093/jmcb/mjad030. J Mol Cell Biol. 2023. PMID: 37127426 Free PMC article.
-
Tim-3 Blockade Elicits Potent Anti-Multiple Myeloma Immunity of Natural Killer Cells.Front Oncol. 2022 Feb 25;12:739976. doi: 10.3389/fonc.2022.739976. eCollection 2022. Front Oncol. 2022. PMID: 35280800 Free PMC article.
-
Galectin-9: diverse roles in skin disease.Front Allergy. 2025 Jul 16;6:1614277. doi: 10.3389/falgy.2025.1614277. eCollection 2025. Front Allergy. 2025. PMID: 40741333 Free PMC article. Review.
-
Controversies in the Design of Strategies for the Cure of HIV Infection.Pathogens. 2023 Feb 15;12(2):322. doi: 10.3390/pathogens12020322. Pathogens. 2023. PMID: 36839593 Free PMC article. Review.
-
Galectin 9 Levels as a Potential Predictor of Intact HIV Reservoir Decay.J Infect Dis. 2025 Feb 4;231(1):156-164. doi: 10.1093/infdis/jiae426. J Infect Dis. 2025. PMID: 39207259 Free PMC article.
References
-
- Archin NM, Bateson R, Tripathy MK, Crooks AM, Yang KH, Dahl NP, Kearney MF, Anderson EM, Coffin JM, Strain MC, Richman DD, Robertson KR, Kashuba AD, Bosch RJ, Hazuda DJ, Kuruc JD, Eron JJ, Margolis DM. 2014. HIV-1 expression within resting CD4+ T cells after multiple doses of vorinostat. J Infect Dis 210:728–735. doi:10.1093/infdis/jiu155. - DOI - PMC - PubMed
-
- Rasmussen TA, Tolstrup M, Brinkmann CR, Olesen R, Erikstrup C, Solomon A, Winckelmann A, Palmer S, Dinarello C, Buzon M, Lichterfeld M, Lewin SR, Østergaard L, Søgaard OS. 2014. Panobinostat, a histone deacetylase inhibitor, for latent-virus reactivation in HIV-infected patients on suppressive antiretroviral therapy: a phase 1/2, single group, clinical trial. Lancet HIV 1:e13–e21. doi:10.1016/S2352-3018(14)70014-1. - DOI - PubMed
-
- Elliott JH, McMahon JH, Chang CC, Lee SA, Hartogensis W, Bumpus N, Savic R, Roney J, Hoh R, Solomon A, Piatak M, Gorelick RJ, Lifson J, Bacchetti P, Deeks SG, Lewin SR. 2015. Short-term administration of disulfiram for reversal of latent HIV infection: a phase 2 dose-escalation study. Lancet HIV 2:e520–e529. doi:10.1016/S2352-3018(15)00226-X. - DOI - PMC - PubMed
-
- Søgaard OS, Graversen ME, Leth S, Olesen R, Brinkmann CR, Nissen SK, Kjaer AS, Schleimann MH, Denton PW, Hey-Cunningham WJ, Koelsch KK, Pantaleo G, Krogsgaard K, Sommerfelt M, Fromentin R, Chomont N, Rasmussen TA, Østergaard L, Tolstrup M. 2015. The depsipeptide romidepsin reverses HIV-1 latency in vivo. PLoS Pathog 11:e1005142. doi:10.1371/journal.ppat.1005142. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

