Aberrant RhoA activation in macrophages increases senescence-associated secretory phenotypes and ectopic calcification in muscular dystrophic mice
- PMID: 33361519
- PMCID: PMC7803538
- DOI: 10.18632/aging.202413
Aberrant RhoA activation in macrophages increases senescence-associated secretory phenotypes and ectopic calcification in muscular dystrophic mice
Abstract
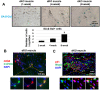
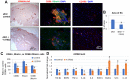
Duchenne Muscular Dystrophy (DMD) patients often suffer from both muscle wasting and osteoporosis. Our previous studies have revealed reduced regeneration potential in skeletal muscle and bone, concomitant with ectopic calcification of soft tissues in double knockout (dKO, dystrophin-/-; utrophin-/-) mice, a severe murine model for DMD. We found significant involvement of RhoA/ROCK (Rho-Associated Protein Kinase) signaling in mediating ectopic calcification of muscles in dKO mice. However, the cellular identity of these RhoA+ cells, and the role that RhoA plays in the chronic inflammation-associated pathologies has not been elucidated. Here, we report that CD68+ macrophages are highly prevalent at the sites of ectopic calcification of dKO mice, and that these macrophages highly express RhoA. Macrophages from dKO mice feature a shift towards a more pro-inflammatory M1 polarization and an increased expression of various senescence-associated secretory phenotype (SASP) factors that was reduced with the RhoA/ROCK inhibitor Y-27632. Further, systemic inhibition of RhoA activity in dKO mice led to reduced number of RhoA+/CD68+ cells, as well as a reduction in fibrosis and ectopic calcification. Together, these data revealed that RhoA signaling may be a key regulator of imbalanced mineralization in the dystrophic musculoskeletal system and consequently a therapeutic target for the treatment of DMD or other related muscle dystrophies.
Keywords: cellular senescence; chronic inflammation; heterotopic ossification; muscle dystrophy; muscle stem cell.
Conflict of interest statement
Figures




Similar articles
-
RhoA mediates defective stem cell function and heterotopic ossification in dystrophic muscle of mice.FASEB J. 2013 Sep;27(9):3619-31. doi: 10.1096/fj.13-233460. Epub 2013 May 23. FASEB J. 2013. PMID: 23704088 Free PMC article.
-
Total Absence of Dystrophin Expression Exacerbates Ectopic Myofiber Calcification and Fibrosis and Alters Macrophage Infiltration Patterns.Am J Pathol. 2020 Jan;190(1):190-205. doi: 10.1016/j.ajpath.2019.09.021. Epub 2019 Nov 11. Am J Pathol. 2020. PMID: 31726040
-
Alterations in Notch signalling in skeletal muscles from mdx and dko dystrophic mice and patients with Duchenne muscular dystrophy.Exp Physiol. 2014 Apr;99(4):675-87. doi: 10.1113/expphysiol.2013.077255. Epub 2014 Jan 17. Exp Physiol. 2014. PMID: 24443351
-
Understanding the process of fibrosis in Duchenne muscular dystrophy.Biomed Res Int. 2014;2014:965631. doi: 10.1155/2014/965631. Epub 2014 May 4. Biomed Res Int. 2014. PMID: 24877152 Free PMC article. Review.
-
Macrophages in the Context of Muscle Regeneration and Duchenne Muscular Dystrophy.Int J Mol Sci. 2024 Sep 27;25(19):10393. doi: 10.3390/ijms251910393. Int J Mol Sci. 2024. PMID: 39408722 Free PMC article. Review.
Cited by
-
The functional role of cellular senescence during vascular calcification in chronic kidney disease.Front Endocrinol (Lausanne). 2024 Jan 22;15:1330942. doi: 10.3389/fendo.2024.1330942. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 38318291 Free PMC article. Review.
-
Targeting EP2 Receptor Improves Muscle and Bone Health in Dystrophin-/-/Utrophin-/- Double-Knockout Mice.Cells. 2025 Jan 14;14(2):116. doi: 10.3390/cells14020116. Cells. 2025. PMID: 39851544 Free PMC article.
-
Epithelial Cell Transformation and Senescence as Indicators of Genome Aging: Current Advances and Unanswered Questions.Int J Mol Sci. 2021 Jul 14;22(14):7544. doi: 10.3390/ijms22147544. Int J Mol Sci. 2021. PMID: 34299168 Free PMC article. Review.
-
Identification of novel macrophages and bone morphogenetic protein signals causing ectopic calcification and impairing muscle regeneration.iScience. 2025 Jun 9;28(7):112841. doi: 10.1016/j.isci.2025.112841. eCollection 2025 Jul 18. iScience. 2025. PMID: 40612505 Free PMC article.
-
Senolytic elimination of senescent macrophages restores muscle stem cell function in severely dystrophic muscle.Aging (Albany NY). 2022 Sep 8;14(19):7650-7661. doi: 10.18632/aging.204275. Epub 2022 Sep 8. Aging (Albany NY). 2022. PMID: 36084954 Free PMC article.
References
-
- Hsu JD. Extremity fractures in children with neuromuscular disease. Johns Hopkins Med J. 1979; 145:89–93. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

