Schistosoma mansoni eggs induce Wnt/β-catenin signaling and activate the protooncogene c-Jun in human and hamster colon
- PMID: 33361772
- PMCID: PMC7758332
- DOI: 10.1038/s41598-020-79450-4
Schistosoma mansoni eggs induce Wnt/β-catenin signaling and activate the protooncogene c-Jun in human and hamster colon
Abstract
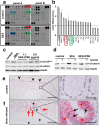
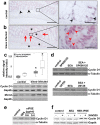
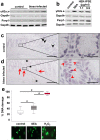
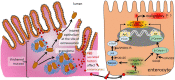
Schistosomiasis (bilharzia) is a neglected tropical disease caused by parasitic flatworms of the genus Schistosoma, with considerable morbidity in parts of the Middle East, South America, Southeast Asia, in sub-Saharan Africa, and particularly also in Europe. The WHO describes an increasing global health burden with more than 290 million people threatened by the disease and a potential to spread into regions with temperate climates like Corsica, France. The aim of our study was to investigate the influence of S. mansoni infection on colorectal carcinogenic signaling pathways in vivo and in vitro. S. mansoni infection, soluble egg antigens (SEA) and the Interleukin-4-inducing principle from S. mansoni eggs induce Wnt/β-catenin signaling and the protooncogene c-Jun as well as downstream factor Cyclin D1 and markers for DNA-damage, such as Parp1 and γH2a.x in enterocytes. The presence of these characteristic hallmarks of colorectal carcinogenesis was confirmed in colon biopsies from S. mansoni-infected patients demonstrating the clinical relevance of our findings. For the first time it was shown that S. mansoni SEA may be involved in the induction of colorectal carcinoma-associated signaling pathways.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Schistosomiasis—fact sheet. https://www.who.int/news-room/fact-sheets/detail/schistosomiasis (2019).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

