Therapeutic response monitoring after targeted therapy in an orthotopic rat model of hepatocellular carcinoma using contrast-enhanced ultrasound: Focusing on inter-scanner, and inter-operator reproducibility
- PMID: 33362203
- PMCID: PMC7757904
- DOI: 10.1371/journal.pone.0244304
Therapeutic response monitoring after targeted therapy in an orthotopic rat model of hepatocellular carcinoma using contrast-enhanced ultrasound: Focusing on inter-scanner, and inter-operator reproducibility
Abstract
Purpose: To assess therapeutic response monitoring after targeted therapy in an orthotopic rat model of hepatocellular carcinoma (HCC) using CEUS with focusing on inter-scanner and inter-operator reproducibility.
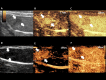
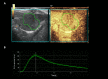
Materials and methods: For reproducibility, CEUS was performed using two different US scanners by two operators in sixteen rat models of HCC. Using perfusion analysis software (VueBox ®), eleven parameters were collected, and intra-class correlation coefficient (ICC) was used to analyze reproducibility. Then seventeen rat models of HCC were divided into treatment group (n = 8, 30 mg/kg/day sorafenib for five days) and control group (n = 9). CEUS was performed at baseline and 14 days after first treatment, and changes of perfusion parameters were analyzed.
Results: In treatment group, CEUS perfusion parameters showed a significant change. The peak enhancement (PE, 2.50 x103±1.68 x103 vs 5.55x102±4.65x102, p = 0.010) and wash-in and wash out AUC (WiWoAUC, 1.07x105±6.48 x104 vs 2.65x104±2.25x104, p = 0.009) had significantly decreased two weeks after treatment. On the contrary, control group did not show a significant change, including PE (1.15 x103±7.53x102 vs 9.43x102± 7.81 x102, p = 0.632) and WiWoAUC (5.09 x104±3.25x104 vs 5.92 x104±3.20x104, p = 0.646). For reproducibility, the various degrees of inter-scanner reproducibility were from poor to good (ICC: <0.01-0.63). However, inter-operator reproducibility of important perfusion parameters, including WiAUC, WoAUC, and WiWoAUC, ranged from fair to excellent (ICC: 0.59-0.93) in a different scanner.
Conclusion: Our results suggest that CEUS is useful for assessment of the treatment response after targeted therapy and with fair to excellent inter-operator reproducibility.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Potential application of dynamic contrast enhanced ultrasound in predicting microvascular invasion of hepatocellular carcinoma.Clin Hemorheol Microcirc. 2021;77(4):461-469. doi: 10.3233/CH-201085. Clin Hemorheol Microcirc. 2021. PMID: 33459703
-
Savitzky-Golay filter based contrast-enhanced ultrasound quantification in hepatic tumors: Methodology and its correlation with tumor angiogenesis.Clin Hemorheol Microcirc. 2019;73(2):271-282. doi: 10.3233/CH-180432. Clin Hemorheol Microcirc. 2019. PMID: 30103307
-
The diagnositic value of dynamic contrast-enhanced ultrasound for evaluation of tissue oxygen status in rat hepatoma model.BMC Gastroenterol. 2024 Nov 23;24(1):424. doi: 10.1186/s12876-024-03523-1. BMC Gastroenterol. 2024. PMID: 39578733 Free PMC article.
-
Comparison of dynamic contrast-enhanced magnetic resonance imaging and contrast-enhanced ultrasound for evaluation of the effects of sorafenib in a rat model of hepatocellular carcinoma.Magn Reson Imaging. 2019 Apr;57:156-164. doi: 10.1016/j.mri.2018.11.012. Epub 2018 Nov 19. Magn Reson Imaging. 2019. PMID: 30465870
-
VueBox® for quantitative analysis of contrast-enhanced ultrasound in liver tumors1.Clin Hemorheol Microcirc. 2022;80(4):473-486. doi: 10.3233/CH-211261. Clin Hemorheol Microcirc. 2022. PMID: 34897079 Review.
Cited by
-
Quantitative analysis of contrast-enhanced ultrasonography in rat models of hepatic acute graft-versus-host disease.Quant Imaging Med Surg. 2023 Aug 1;13(8):4908-4918. doi: 10.21037/qims-22-1145. Epub 2023 May 22. Quant Imaging Med Surg. 2023. PMID: 37581062 Free PMC article.
-
A Comprehensive and Repeatable Contrast-Enhanced Ultrasound Quantification Approach for Clinical Evaluations of Tumor Blood Flow.Invest Radiol. 2025 Apr 1;60(4):281-290. doi: 10.1097/RLI.0000000000001127. Epub 2024 Oct 9. Invest Radiol. 2025. PMID: 39418656
References
-
- Guibal A, Taillade L, Mule S, Comperat E, Badachi Y, Golmard JL, et al. Noninvasive contrast-enhanced US quantitative assessment of tumor microcirculation in a murine model: effect of discontinuing anti-VEGF therapy. Radiology. 2010;254(2):420–9. Epub 2010/01/23. 10.1148/radiol.09090728 . - DOI - PubMed
-
- Lamuraglia M, Bridal SL, Santin M, Izzi G, Rixe O, Paradiso A, et al. Clinical relevance of contrast-enhanced ultrasound in monitoring anti-angiogenic therapy of cancer: current status and perspectives. Crit Rev Oncol Hematol. 2010;73(3):202–12. Epub 2009/06/24. 10.1016/j.critrevonc.2009.06.001 . - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

