Proton-dependent inhibition, inverted voltage activation, and slow gating of CLC-0 Chloride Channel
- PMID: 33362212
- PMCID: PMC7757909
- DOI: 10.1371/journal.pone.0240704
Proton-dependent inhibition, inverted voltage activation, and slow gating of CLC-0 Chloride Channel
Abstract
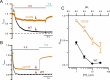
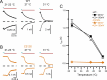
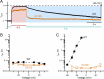
CLC-0, a prototype Cl- channel in the CLC family, employs two gating mechanisms that control its ion-permeation pore: fast gating and slow gating. The negatively-charged sidechain of a pore glutamate residue, E166, is known to be the fast gate, and the swinging of this sidechain opens or closes the pore of CLC-0 on the millisecond time scale. The other gating mechanism, slow gating, operates with much slower kinetics in the range of seconds to tens or even hundreds of seconds, and it is thought to involve still-unknown conformational rearrangements. Here, we find that low intracellular pH (pHi) facilitates the closure of the CLC-0's slow gate, thus generating current inhibition. The rate of low pHi-induced current inhibition increases with intracellular H+ concentration ([H+]i)-the time constants of current inhibition by low pHi = 4.5, 5.5 and 6 are roughly 0.1, 1 and 10 sec, respectively, at room temperature. In comparison, the time constant of the slow gate closure at pHi = 7.4 at room temperature is hundreds of seconds. The inhibition by low pHi is significantly less prominent in mutants favoring the slow-gate open state (such as C212S and Y512A), further supporting the fact that intracellular H+ enhances the slow-gate closure in CLC-0. A fast inhibition by low pHi causes an apparent inverted voltage-dependent activation in the wild-type CLC-0, a behavior similar to those in some channel mutants such as V490W in which only membrane hyperpolarization can open the channel. Interestingly, when V490W mutation is constructed in the background of C212S or Y512A mutation, the inverted voltage-dependent activation disappears. We propose that the slow kinetics of CLC-0's slow-gate closure may be due to low [H+]i rather than due to the proposed large conformational change of the channel protein. Our results also suggest that the inverted voltage-dependent opening observed in some mutant channels may result from fast closure of the slow gate by the mutations.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures






Similar articles
-
Insights into CLC-0's Slow-Gating from Intracellular Proton Inhibition.Int J Mol Sci. 2024 Jul 16;25(14):7796. doi: 10.3390/ijms25147796. Int J Mol Sci. 2024. PMID: 39063037 Free PMC article.
-
Voltage-dependent and -independent titration of specific residues accounts for complex gating of a ClC chloride channel by extracellular protons.J Physiol. 2009 Apr 1;587(Pt 7):1387-400. doi: 10.1113/jphysiol.2008.167353. Epub 2009 Jan 19. J Physiol. 2009. PMID: 19153159 Free PMC article.
-
The role of protons in fast and slow gating of the Torpedo chloride channel ClC-0.Eur Biophys J. 2010 May;39(6):869-75. doi: 10.1007/s00249-008-0393-x. Epub 2009 Jan 9. Eur Biophys J. 2010. PMID: 19132363
-
Coupling gating with ion permeation in ClC channels.Sci STKE. 2003 Jun 24;2003(188):pe23. doi: 10.1126/stke.2003.188.pe23. Sci STKE. 2003. PMID: 12824475 Review.
-
Emerging issues of connexin channels: biophysics fills the gap.Q Rev Biophys. 2001 Aug;34(3):325-472. doi: 10.1017/s0033583501003705. Q Rev Biophys. 2001. PMID: 11838236 Review.
Cited by
-
Insights into CLC-0's Slow-Gating from Intracellular Proton Inhibition.Int J Mol Sci. 2024 Jul 16;25(14):7796. doi: 10.3390/ijms25147796. Int J Mol Sci. 2024. PMID: 39063037 Free PMC article.
-
Biophysical and Pharmacological Insights to CLC Chloride Channels.Handb Exp Pharmacol. 2024;283:1-34. doi: 10.1007/164_2022_594. Handb Exp Pharmacol. 2024. PMID: 35768555

