A cost/benefit analysis of clinical trial designs for COVID-19 vaccine candidates
- PMID: 33362278
- PMCID: PMC7757868
- DOI: 10.1371/journal.pone.0244418
A cost/benefit analysis of clinical trial designs for COVID-19 vaccine candidates
Abstract
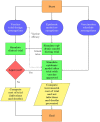
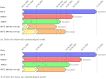
We compare and contrast the expected duration and number of infections and deaths averted among several designs for clinical trials of COVID-19 vaccine candidates, including traditional and adaptive randomized clinical trials and human challenge trials. Using epidemiological models calibrated to the current pandemic, we simulate the time course of each clinical trial design for 756 unique combinations of parameters, allowing us to determine which trial design is most effective for a given scenario. A human challenge trial provides maximal net benefits-averting an additional 1.1M infections and 8,000 deaths in the U.S. compared to the next best clinical trial design-if its set-up time is short or the pandemic spreads slowly. In most of the other cases, an adaptive trial provides greater net benefits.
Conflict of interest statement
P.H., K.S., and C.W. report no conflicts. L.I. is an employee of the biotech company Seqirus and receives salary and company stock as part of compensation. A.L. reports personal investments in private biotech companies, biotech venture capital funds, and mutual funds. A.L. is a co-founder and partner of QLS Advisors, a healthcare analytics and consulting company; an advisor to BrightEdge Ventures; a director of BridgeBio Pharma, Roivant Sciences, and Annual Reviews; chairman emeritus and senior advisor to AlphaSimplex Group; and a member of the Board of Overseers at Beth Israel Deaconess Medical Center and the NIH’s National Center for Advancing Translational Sciences Advisory Council and Cures Acceleration Network Review Board. During the most recent six-year period, A.L. has received speaking/consulting fees, honoraria, or other forms of compensation from: AIG, AlphaSimplex Group, BIS, BridgeBio Pharma, Citigroup, Chicago Mercantile Exchange, Financial Times, FONDS Professionell, Harvard University, IMF, National Bank of Belgium, Q Group, Roivant Sciences, Scotia Bank, State Street Bank, University of Chicago, and Yale University. Funding support from the MIT Laboratory for Financial Engineering is gratefully acknowledged, but no direct funding was received for this study, no commercial funding was provided or solicited for this study, and no funding bodies had any role in study design, data collection and analysis, decision to publish, or preparation of this manuscript. The authors were personally salaried by their institutions during the period of writing (though no specific salary was set aside or given for the writing of this manuscript). This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures
References
-
- Deutsch J. Von der Leyen: Life won’t return to normal until vaccine. Politico. 2020.
-
- Abutaleb Y, Dawsey J, McGinley L, Johnson CY. Trump pushing officials to speed up already-ambitious coronavirus vaccine timeline. Washington Post. 2020.
-
- Lo AW, Siah KW, Wong CH. Estimating Probabilities of Success of Vaccine and Other Anti-Infective Therapeutic Development Programs. Harvard Data Science Review. 2020;. 10.1162/99608f92.e0c150e8 - DOI
-
- Burton TM. FDA to Require Proof Virus Vaccine Is Effective Before Approving Its Use. The Wall Street Journal. 2020.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

