The effects of COVID-19 on IBD prescribing and service provision in a UK tertiary centre
- PMID: 33362435
- PMCID: PMC7753474
- DOI: 10.1002/ygh2.433
The effects of COVID-19 on IBD prescribing and service provision in a UK tertiary centre
Abstract
Background: To quantify the effects of COVID-19 on our inflammatory bowel disease (IBD) unit, including service provision, prescribing practices and use of therapeutic drug monitoring (TDM).
Methods: We performed a single centre retrospective observational cohort study. Data was extracted from our IBD database, electronic patient records and radiology/endoscopy reporting systems between 16/3/20-17/4/20 and the corresponding period in 2019.
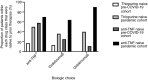
Results: A similar number of patients commenced biologic therapy before COVID-19 (n = 37) and during the pandemic (n = 36). Patients in the pre-COVID-19 cohort were older (median 36 vs 29 years, P = 0.009) with a longer median disease duration (9.3 vs 5.2 years, P = 0.02). During COVID-19 there was a nonsignificant increase in prescribing of vedolizumab (8/37, 22% vs 14/36, 39%, P = 0.13) and a higher proportion of patients were anti-TNF-naïve (3/17, 18% vs 18/24, 74%, P = 0.0004). There was a reduction in use of concomitant immunomodulators (22/29, 76% vs 4/34, 12%, P < 0.0001) and increased biologic use in thiopurine-naïve patients (3/37, 8% vs 15/36, 42%, P = 0.001). Use of TDM fell by 75% (240 vs 59 tests). Outpatient appointments fell by 68% and were conducted via telemedicine. MRI scanning, endoscopy, luminal surgery and inpatient numbers fell by 87%, 85%, 100% and 82% respectively. IBD Clinical Nurse Specialist and Pharmacist helpline contacts increased by 76% and 228% respectively.
Conclusions: We observed prescribing differences during COVID-19, bypassing the initiation of immunomodulators and/or anti-TNF therapy in favour of vedolizumab with a reduction in immunomodulator prescribing. We also observed a rapid reorganisation of service provision, including a shift towards telemedicine and online solutions.
Keywords: biologics; immunosuppression; inflammatory bowel disease.
© 2020 John Wiley & Sons Ltd.
Figures

References
-
- Endoscopy JAGoG . Advice for Endoscopy Teams during COVID‐19. BSG. 2020.
-
- British Society of Gastroenterology . BSG guide for IBD patients during COVID‐19 pandemic. 2020.
-
- National Institute for Health and Care Excellence . Infliximab and adalimumab for the treatment of Crohn's disease. NICE; 2010. [updated 2020 Apr] (Technology Appraisal guidance [TA187). https://wwwniceorguk/guidance/ta187/2010. Accessed May 2010
-
- Kalb RE, Fiorentino DF, Lebwohl MG, et al. Risk of Serious Infection With Biologic and Systemic Treatment of Psoriasis: Results From the Psoriasis Longitudinal Assessment and Registry (PSOLAR). JAMA Dermatology. 2015;151:961‐969. - PubMed
LinkOut - more resources
Full Text Sources
