Role of Nuclear Imaging to Understand the Neural Substrates of Brain Disorders in Laboratory Animals: Current Status and Future Prospects
- PMID: 33362486
- PMCID: PMC7759612
- DOI: 10.3389/fnbeh.2020.596509
Role of Nuclear Imaging to Understand the Neural Substrates of Brain Disorders in Laboratory Animals: Current Status and Future Prospects
Abstract
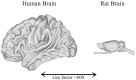
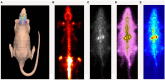
Molecular imaging, which allows the real-time visualization, characterization and measurement of biological processes, is becoming increasingly used in neuroscience research. Scintigraphy techniques such as single photon emission computed tomography (SPECT) and positron emission tomography (PET) provide qualitative and quantitative measurement of brain activity in both physiological and pathological states. Laboratory animals, and rodents in particular, are essential in neuroscience research, providing plenty of models of brain disorders. The development of innovative high-resolution small animal imaging systems together with their radiotracers pave the way to the study of brain functioning and neurotransmitter release during behavioral tasks in rodents. The assessment of local changes in the release of neurotransmitters associated with the performance of a given behavioral task is a turning point for the development of new potential drugs for psychiatric and neurological disorders. This review addresses the role of SPECT and PET small animal imaging systems for a better understanding of brain functioning in health and disease states. Brain imaging in rodent models faces a series of challenges since it acts within the boundaries of current imaging in terms of sensitivity and spatial resolution. Several topics are discussed, including technical considerations regarding the strengths and weaknesses of both technologies. Moreover, the application of some of the radioligands developed for small animal nuclear imaging studies is discussed. Then, we examine the changes in metabolic and neurotransmitter activity in various brain areas during task-induced neural activation with special regard to the imaging of opioid, dopaminergic and cannabinoid receptors. Finally, we discuss the current status providing future perspectives on the most innovative imaging techniques in small laboratory animals. The challenges and solutions discussed here might be useful to better understand brain functioning allowing the translation of preclinical results into clinical applications.
Keywords: PET - positron emission tomography; SPECT; behavioral neuroscience; brain disorders; neuroimaging; small animal imaging.
Copyright © 2020 D'Elia, Schiavi, Soluri, Massari, Soluri and Trezza.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Acton P. D., Hou C., Kung M. P., Plossl K., Keeney C. L., Kung H. F. (2002b). Occupancy of dopamine D2 receptors in the mouse brain measured using ultra-high-resolution single-photon emission tomography and [123]IBF. Eur. J. Nucl. Med. Mol. Imaging 29, 1507–1515. 10.1007/s00259-002-0903-5 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

