Validation and Diagnostic Performance of a CFD-Based Non-invasive Method for the Diagnosis of Aortic Coarctation
- PMID: 33362500
- PMCID: PMC7756015
- DOI: 10.3389/fninf.2020.613666
Validation and Diagnostic Performance of a CFD-Based Non-invasive Method for the Diagnosis of Aortic Coarctation
Abstract
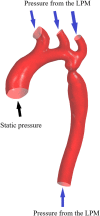
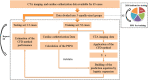
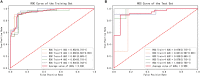
Purpose: The clinical diagnosis of aorta coarctation (CoA) constitutes a challenge, which is usually tackled by applying the peak systolic pressure gradient (PSPG) method. Recent advances in computational fluid dynamics (CFD) have suggested that multi-detector computed tomography angiography (MDCTA)-based CFD can serve as a non-invasive PSPG measurement. The aim of this study was to validate a new CFD method that does not require any medical examination data other than MDCTA images for the diagnosis of CoA. Materials and methods: Our study included 65 pediatric patients (38 with CoA, and 27 without CoA). All patients underwent cardiac catheterization to confirm if they were suffering from CoA or any other congenital heart disease (CHD). A series of boundary conditions were specified and the simulated results were combined to obtain a stenosis pressure-flow curve. Subsequently, we built a prediction model and evaluated its predictive performance by considering the AUC of the ROC by 5-fold cross-validation. Results: The proposed MDCTA-based CFD method exhibited a good predictive performance in both the training and test sets (average AUC: 0.948 vs. 0.958; average accuracies: 0.881 vs. 0.877). It also had a higher predictive accuracy compared with the non-invasive criteria presented in the European Society of Cardiology (ESC) guidelines (average accuracies: 0.877 vs. 0.539). Conclusion: The new non-invasive CFD-based method presented in this work is a promising approach for the accurate diagnosis of CoA, and will likely benefit clinical decision-making.
Keywords: aortic coarctation; congenital heart disease; hydrodynamics; multidetector computed tomography angiography; non-invasive assessment.
Copyright © 2020 Lu, Lin, Zhang, Chen, Wei, Li, Du, Xie, Yu, Xie and Liu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The reviewer QT declared a past co-authorship with the authors (XX, RZ, HL) to the handling editor.
Figures



Similar articles
-
Clinical validation and assessment of aortic hemodynamics using computational fluid dynamics simulations from computed tomography angiography.Biomed Eng Online. 2018 May 2;17(1):53. doi: 10.1186/s12938-018-0485-5. Biomed Eng Online. 2018. PMID: 29720173 Free PMC article.
-
MRI-based computational fluid dynamics for diagnosis and treatment prediction: clinical validation study in patients with coarctation of aorta.J Magn Reson Imaging. 2015 Apr;41(4):909-16. doi: 10.1002/jmri.24639. Epub 2014 Apr 11. J Magn Reson Imaging. 2015. PMID: 24723299
-
Computational fluid dynamics simulation to evaluate aortic coarctation gradient with contrast-enhanced CT.Comput Methods Biomech Biomed Engin. 2015 Aug;18(10):1066-1071. doi: 10.1080/10255842.2013.869321. Epub 2014 Jan 27. Comput Methods Biomech Biomed Engin. 2015. PMID: 24460213
-
Diagnosis, imaging and clinical management of aortic coarctation.Heart. 2017 Aug;103(15):1148-1155. doi: 10.1136/heartjnl-2017-311173. Epub 2017 Apr 4. Heart. 2017. PMID: 28377475 Review.
-
Applications of Computational Fluid Dynamics in Congenital Heart Disease: A Review.J Cardiovasc Dev Dis. 2025 Feb 13;12(2):70. doi: 10.3390/jcdd12020070. J Cardiovasc Dev Dis. 2025. PMID: 39997504 Free PMC article. Review.
Cited by
-
A systematic quantification of hemodynamic differences persisting after aortic coarctation repair.Front Bioeng Biotechnol. 2025 Jun 26;13:1539256. doi: 10.3389/fbioe.2025.1539256. eCollection 2025. Front Bioeng Biotechnol. 2025. PMID: 40642532 Free PMC article.
-
Irregular anatomical features can alter hemodynamics in Takayasu arteritis.JVS Vasc Sci. 2023 Aug 24;4:100125. doi: 10.1016/j.jvssci.2023.100125. eCollection 2023. JVS Vasc Sci. 2023. PMID: 37771369 Free PMC article.
-
A comprehensive MRI-based computational model of blood flow in compliant aorta using radial basis function interpolation.Biomed Eng Online. 2024 Jul 23;23(1):69. doi: 10.1186/s12938-024-01251-x. Biomed Eng Online. 2024. PMID: 39039565 Free PMC article.
-
Investigation of Relationship between Hemodynamic and Morphometric Characteristics of Aortas in Pediatric Patients.J Clin Med. 2024 Aug 29;13(17):5141. doi: 10.3390/jcm13175141. J Clin Med. 2024. PMID: 39274354 Free PMC article.
-
Using computed tomography angiography and computational fluid dynamics to study aortic coarctation in different arch morphologies.Front Pediatr. 2023 Jun 27;11:1131025. doi: 10.3389/fped.2023.1131025. eCollection 2023. Front Pediatr. 2023. PMID: 37441569 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

